* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Anomalous Pulmonary Vein Serving as Collateral Channel in Aortic
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
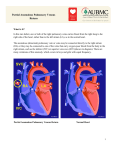
ANOMALOUS PULMONARY VEIN AS COLLATERAL IN AORTIC STENOSIS Anomalous Pulmonary Vein Serving as Collateral Channel in Aortic Stenosis with Hypoplastic Left Ventricle and Endocardial Fibroelastosis* Cad E. Hunt, M.D., S d y a m r a y a ~Rao, M.B., B.S., James H. MoUer, M.D. and Jesse E. Edwards, M.D. In an infant mW aortic s t e n d and hypoph& left ventricle, obstmction was PssociPted WW a pulmonary venous collated channel to the left iDMMlinrtc v e h Viwutlzedio~udBveIKmEdrrnnd was atmnPr b total anomP1011~pohnonaty venous connection. n atresia, either of the aortic or of the mitral Ithrough valve, the pulmonary venous return must pass an abnormal pathway to reach the systemic circulation. In each condition, the usual route For editorial comment, see page 113. for escape of blood from the left side of the heart into the right side is through an interatrial communication. Uncommonly, as when the foramen ovale is either sealed or unusually small, the channel for flow of pulmonary venous blood to the right side of the heart is through an anomalous vein. The latter may originate either from a pulmonary vein1 or from the left a t r i ~ m . In ~ , ~either instance, it terminates in a systemic vein. (Such a vein onginating at the atrium has been termed levoatriocardinal vein.) When an anomalous pulmonary vein serves as the main channel for flow of blood from the lungs to the right side of the circulation, angiographic demonstration of the anomalous channel may be obtained. The angiograms may be falsely interpreted as representing the enti9 total anomalous pulmonary venous connection, whereas, in fact, the vein demonstrated angiographically is simply a collateral channel incident to serious intracardiac obstruction. The purpose of this report is to describe a case of hypoplasia of the left ventricle with endocardial fibroelastosis and aortic stenosis (which, in some respects, is functionally like aortic and/or mitral atresia) in which an anomalous vein extended from the left upper pulmonary vein to the left innominate vein. The channel served as a principal channel for the flow of blood from the pulmonary 'From the Departments of Pediatrics and Pathology, The University of Minnesota, Minnea lis, Minnesota and the Departpent of Pathology, The &rles T. Miller Hospital. St. Paul. Minnesota. This study was supported Public Health Service Research Grant 5 RO1 H E O h and Research Training Grant 5 TO1 HE05SIO from the National Heart Institute. veins to the right side of the heart and was demonstrated angiocardiographically. We wish to indicate the similar angiographic features between total anomalous pulmonary venous connection and the anomalous vein in conditions with left-sided intracardiac obstruction and means of distinguishing them by catheterization and angiographic techniques. The patient was a full-term boy delivered by cesarean section. At birth, he required resuscitation. He responded well and was discharged from the hospital at six days of age in apparent good health. Over the next six weeks, however, the infant exhibited rapid, noisy respirations, poor feeding, and upon crying, peripheral cyanosis. When six weeks old, the infant was thought to be in a state of congestive heart failure and was referred for evaluation. Physical examination on admission revealed a mildly cyanotic infant in respiratory distress. The cardiac rate was 160 per minute and respiratory rate was 80 per minute. Values for blood pressures measured simultaneously by the flush method were equal in an arm and leg. The lungs were clear to auscultation. Examination of the heart revealed no thrill or heave. The first sound was split with an ejection click along the lower left sternal border. A grade I/VI systolic ejection murmur was present at the base and radiated to the upper back. The second sound was split and the pulmonic component was moderately accentuated. The edge of the liver was palpable 4 cm below the right costal margin. The spleen was not palpable. Examination of the extremities revealed weakly palpable pulses with cyanotic nail beds. No differential cyanosis was present. The concentration of hemoglobin was 17.8 grams per 100 milliliters of blood. The electrocardiogram revealed s normal QRS axis (+105"), right atrial enlargement, and left ventricular hypertrophy with strain. Thoracic roentgenograms revealed marked cardiomegaly with prominent pulmonary arterial vasculature (Fig 1). The initial clinical impression was tricuspid valvular atresia. Right-sided cardiac catheterization was performed. The tip of the catheter could he advanced intn the right FIGURE1. Roentgenogram of thorax when patient was six weeks old. Cardiomegaly. In the original films, the pulmonary vasculature appeared prominent. CHEST, VOL. 57, NO. 2, FEBRUARY 1970 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21488/ on 06/17/2017 HUNT ET AL ventricle and then through a patent ductus into the descending aorta. The pressure in the latter compartment was low (63/45, mean = 58 mm Hg). A significant increase in peak systolic pressure was found as the catheter was withdrawn to the main pulmonary artery (118/57, mean = 75 mm Hg). The right ventricular pressure was also markedly elevated (118/0-20). From the right atrium, the catheter was advanced across a patent foramen ovale into the left atrium. From the latter chamber, the catheter was advanced into the left upper pulmonary vein and, from that vein, into an anomalous vein which coursed superiorly toward the neck. The mean pressure in each atrium was elevated (19 mm Hg). Data for oxygen saturation indicated three features of significance as follows: (a) pulmonary venous desaturation (76 percent); (b) a right-to-left shunt through the ductus arteriosus; and (c) no site of significant increase in oxygen saturation in the superior vena cava or the right atrium. A right ventriculogram demonstrated an enlarged hypertrophied right ventricle and a "reversing" patent ductus arteriosus. In the levogram phase, pulmonary veins were well visualized and, in addition. a vein running vertically in the left upper mediastinurn was seen. Initially, this vein was thought to represent total anomalous pulmonary venous connection, but the presence of a reversing ductus arteriosus and subsequent filling of the brachiocephalic vessels was felt to be unusual for total anomalous pulmonary venous connection as a primary d y . Them fore, a left atriogram was performed (Fig 2). This study revealed the presence of a small left atrium and reflux of contrast media into the left and right pulmonary veins, indicating that these veins entered the left atrium in a normal fashion. From the region of the junction of the left upper pulmonary vein with the left atrium, an anomalous vein arose and passed superiorly to enter the left innominate vein. A thick left ventricular wall encased a small ventricular cavity. The latter did not change in shape or size throughout the remainder of the angiocardiogram. The studies led to a diagnosis of hypoplastic left heart syndrome with pulmonary venous collateral flow into the left innominate vein. The patient's condition deteriorated and he expired 12 hours after the catheterization. Pathologic Findings: At necropsy, the findings in the heart and vascular system were of greatest interest. The great vessels were normally related. The ascending aorta was relatively narrow, measuring 0.8 cm in diameter, while the pulmonary trunk was somewhat distended and tense. It measured 1.5 cm in diameter. The aortic arch revealed a zone of tubular hypoplasia in the segment between the left common carotid and the left subclavian arteries. In this region, the arch measured 0.4 cm in diameter. The aorta widened to a diameter of about 1 cm distal to the entrance of a widely patent ductus arteriosus. The left ventricle was the site of abnormalities, one of which was hypoplasia of the chamber (Fig 3a). Tbe latter measured 3.5 cm in bight, as from FIGURE 2. Left atriogram in frontal view. a Early phase. Shardy after injedh of conmaterial into the left atrium (LA), there is evidence of reflux into each pulmonary vein and, in addition, an anomalous vein (AV) arises from the left upper pulmonary vein (LUPV). This allows opacification of the It ascends and terminates in the left innominate vein 0. superior vena &va (SVC). No evidence of a left-to-right shunt between the atria. b. Late phase showing opacification of the right atrium (RA) and the right (RV) and left (LV) ventricles. O p d c a t i o n of the left innominate vein 0 and the superior vena cava is still evident. It is concluded that o p d c a t i o n of the right-sided chambers results from delivery of blood by way of the anomalous pulmonary venous and the superior vena caval systems. The opacification of the left ventricle i s a residual effect from direct h w from the left atrium into the hypoplastic left ventricle. There is major hypertrophy of the left ventricular wall around the hypoplastic chamber. CHEST, VOL. 57, NO. 2, FEBRUARY 1970 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21488/ on 06/17/2017 ANOMALOUS PULMONARY VEIN AS COLLATERAL IN AORTIC STENOSIS the aortic valve to the apex, and 1.7 cm in greatest width. The wall of the left ventricle was hypertrophied and measured 0.9 cm in thickness and equaled the thickness of the hypertrophied right ventricular wall. The left ventricular endocardium was grossly thickened by white, firm tissue. This layer measured up to 4 mm in thickness. The aortic valve was stenotic, having the deformity of the unicuspid, unicommissural, congenitally stenotic aortic valve. The d t r a l valvular leaflets were essentially normal, although the chordae were relatively short. The left atrial chamber was of normal size. The endocardium showed a mild degree of thickening. Each of the pulmonary veins joined the left atrial chamber in a normal manner (Fig 3b). From the left upper pulmonary vein, just proximal to its junction with the left atrium, a wide vein measuring about 9 mm in diameter proceeded upward anterior to the left pulmonary hilus in a somewhat medial direction to join the lateral extremity of the left innominate vein. The foramen ovale showed a valvular competent patency with a potential opening of 4 mm in diameter. The valve of the foramen ovale was pressed against the atrial septum and showed minimal herniation toward the right atrium. The gross abnormalities are summarized in Figure 4. Histologic examination of the left ventricular myocardium showed marked thickening of the endocardium by collagenous and elastic fibers, while the myocardial tissue was not remarkable. Examination of the lungs revealed thickening of alveolar septae on the basis of interstitial edema. There was moderate thickening - of the medin of the muscular pulmonary arteries and the proximal segments of the arterioles. Distention of capillaries was also a feature. as was dilatation of lym~hatics in the visceral - pleura and interlobular septa. In the case presented, the primary cardiac malformation was hypoplasia of the left ventricle associated with stenosis of the aortic valve. In this situation, although a patent route existed for the flow of blood through the left side of the heart, the evidence indicates that some of the pulmonary venous return was diverted through the described nnomalous vein into the systemic venous system at the level of the left innominate vein. It is assumed that the nature of the left side of the heart constituted a significant obstruction to pulmonary venous flow. This accounted for the development of a collateral channel from the pulmonary venous system to the systemic veins. In this way, the circulation bears similarity to that observed in patients with aortic andlor mitral valvular atresia. In the present patient, left ventricular output was reduced because of left ventricular inflow obstruction incident to the hypoplasia of the left ventricle with endocardial fibroelastosis and the aortic valve was stenotir. As a result, low pressure was found in the proximal segment of the aorta. Support for this concept comes from the fact that when a right ventriculograrn was performed, some of the contrast material, passing into the aorta, FIGURE3. Gross pathologic findings. a. Left atrium (LA) and left ventricle (LV). The left ventricular chamber is small. The endocardium is grossly thickened. There is hypertrophy of the myocardium. The papillary muscles are covered by thickened endocardium and the chordae are relatively short. The left atrium is not remarkable. The atrial septum is normally formed. b. Hilar aspect of the left lung and related structures. Aorta in badcground. The upper probe has passed from the left atrium (LA) into the left upper pulmonary vein and then into the anomalous pulmonary vein (AV) which ran between the left upper pulmonary vein and the left innominate vein. The lower probe lies in the coronary sinus, which is not remarkable. regurgitated into the ascending aorta to opacify the brachiocephalic vessels. The quati& arises as to the manner of develop CHEST, VOL. 57, NO. 2, FEBRUARY 1970 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21488/ on 06/17/2017 HUNT ET AL FIGURE4. Summary of the gross abnormalities observed. a. Emphasis is made of the hypoplastic left ventricle and stenotic aortic valve associated with moderate hypoplasia of the ascending aorta and a patent ductus arteriosus. b. Emphasis is made on the occurrence of an anomalous vein running between the left upper pulmonary vein (LUPV),below, and the left innominate vein (LIV), above. This channel supplied an effective link in the route for the flow of blood from each lung to the systemic venous system, for ultimate delivery to the right atrium. ment of the anomalous vein which extended between the left upper pulmonary vein and the left innominate vein. It is recognized that in the early stages of pulmonary venous development, the primary connections for drainage extend into two major venous systems, the umbilico-vitelline system (from which the portal venous system is developed) and the cardinal system of veins (from which the systemic veins are developed). Only after secondary development between a protrusion from the heart (common pulmonary vein) and the pulmonary venous system is established, do the primitive connections disappear. In instances such as the one herein reported wherein an obstruction to flow from the pulmonary venous system exists, one or several of the primitive connections may be retained as a collateral channel to carry venous blood from the lungs. It is, therefore, concluded that the anomalous vein observed in the case described represents persistence of a primitive venous connection between the pulmonary venous system and the cardinal system of veins incident to failure of development of a free passage from the lungs through the left side of the heart. If this explanation is valid, it would mean that the left ventricular problem existed from early stages of development of the individual. Such a problem could have resulted either from aortic stenosis alone with secondary left ventricular failure or from intrinsic hypoplasia of the left ventricle with endocardia1 fibroelastosis. Identification of the anomalous channel between the left upper pulmonary vein and the left atrium angiocardiographically brought to mind the possibility that the channel was the anomalous trunk as occurs in the entity total anomalous pulmonary venous connection to the left innominate vein. As has been indicated in this case, this channel had a different explanation and was simply an indication of a serious intracardiac disease. Important points in differential diagnosis between total anomalous venous connection, on one hand, and a collateral vein as that described in association with left-sided obstructive disease as seen here need to be made. In total anomalous pulmonary venous connection, the levels of oxygen saturation, classically, are approximately identical in each of the four cardiac chambers. In our case, although there was desaturation of blood of the left atrium, the values in this position were higher than those in the right atrium and in the right ventricle. Another important factor is that, in total anomalous pulmonary venous connection, reversal of flow through a patent ductus arteriosus is present only when a patent ductus and severe pulmonary venous obstruction exist4 In our case there were signs of reversed flow through the ductus arteriosus. This finding represents an important factor suggesting the presence of an CHEST, VOL. 57, NO. 2, FEBRUARY 1970 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21488/ on 06/17/2017 SUBCUTANEOUS IMPLANTATION OF CANCER obstructive lesion either in the pulmonary veins or beyond the pulmonary circulation. Strong evidence for the obstruction to pulmonary venous flow beyond the pulmonary veins was supplied by the hypoplastic left ventricle. An additional support for disease involving the left side of the heart was seen in the angiocardiogram which showed a reversal of contrast material in the aortic arch from the ductus arteriosus. As suggested earlier, the aortic stenosis was probably an important factor in determining the hemodynamics of the proximal aorta that would underlie this observation. In classk examples of total anomalous pulmonary venous connection, the left side of the heart is normal. Under this circumstance, it is anticipated that a left atriogram would yield essentially normal findings. In our case, in contrast, a left atriogram showed the presence of pulmonary veins joining the left atrium which, of itself, would exclude the diagnosis of total anomalous pulmonary venous connection and, in addition, demonstrated the anomalous channel which is the subject of this report. REFERENCES 1 SHONE, J.D. AND EDWARDS, J.E.: Mitral atresia associatecl with pulmonary venolls anomalies, Brit. Heart J., 26: 241, 1964. 2 BUTLER,H.: Some derivations of the foregut venous plexus of the albino rat, with reference to man, 1. Anat.. 86:95,1952. 3 LUCAS,R.V.. JR., LESTER,R.C.., LILLEHEI,C.W. ~ h m EDWARDS, J.E.: Mitral atresia with levoatriocardinal vein. .4 form of congenital pulmonary venous ohstn~ction. A:~rrr.J . Cardinl.. 9:607, 1962. 4 H a s m m , A.R., PA-, M.N., MOLTHAN,M.E. AM, MILLER,R.A.: Total anomalous pulmonary venous connection with severe pulmonary venous obstruction, a clinical entity, Circulation, 25:916, 1962. diagnosis of pleural disorders. Complications, chiefly pneumothorax and intrapleural bleeding, have been unusual and almost always of minor extent. Subcutaneous implantation of cancer along the needle tract has been reported only once before.= This paper describes a second case. A 42-year-old housewife underwent radical mastectomy in November 1984 for scirrhous carcinoma of the right breast. Postoperatively, she received Cobalt-@ teletherapy which was complicated by fibrosis of the right upper lung and pericardial e h i o n , the latter subsiding spontaneously. She remained asymptomatic until August 1966, when she began to note progressively severe dyspnea. A roentgenogram of the chest showed bilateral pleural e h i o n s and densities radiating peripherally from both hilar areas compatible with lymphatic spread of tumor. Thoracentesis and biopsy of the right parietal pleura were performed with a Cope needle. Both cytological and cell block examinations of the fluid revealed malignant cells, and the biopsy showed a cluster of similar cells compatible with metastatic breast carcinoma. Identical malignant cells also were noted in fluid removed subsequently by a left thoracentesis which was performed without biopsy. Thio-TEPA was instilled in each pleural space, and therapy with testosterone ,was begun. (~ilateraloophorectomy had been performed elsewhere in September 1963, for a benign disorder). No changes occurred in the bilateral pleural densities during the ensuing year, but there was clinical and roentgenographic evidence of increasing lymphatic spread of tumor in both lungs. Further palliative therapy incliided stilbestrol, radiotherapy of metastatic lesions of the spine and pelvis, and three courses of 5-fluorouracil. In March 1967, six months after thoracentesis and needle biopsy, sht= developed a firm, tender nodule measur- Reprint requests. Dr. esse E. Edwards, Charles T. Miller Hospital, 125 West Col ege Avenue, St. Paul 55102 1 Subcutaneous Implantation of Cancer: A Rare Complication of Pleural Biopsy Frederick L. J M , Jr., M.D.,F.C.C.P.. A ease of wbcutaneous implantation and growtb of mammary cancer fonorPing needle biopsy of the parietal plemra is reported. Tbis is a rare complication wbieb does not Meet the prognh of patients with m w pleural efhsions. Fear of such local spread of tumor &odd not serve as a contraindication to p l d biopsy. S i n c e DeFrancisl introduced needle biopsy of the parietal pleura in 1955, this technique has been universally accepted and employed in the "From the Department of Internal ~ e d i c i n e , Geisinger Medical Center, Dandle, Pennsylvania. FIGURE1. Subcutaneous nodule at site of needle biapsy of parietal pleura Arrow points to indsional scar. CHEST, WL. 57, NO. 2, FEBRUARY 1970 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21488/ on 06/17/2017