* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermodynamics - SeyedAhmad.com
Survey
Document related concepts
Copper in heat exchangers wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat capacity wikipedia , lookup
Equipartition theorem wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Conservation of energy wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Internal energy wikipedia , lookup
History of thermodynamics wikipedia , lookup
Transcript
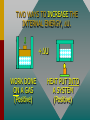
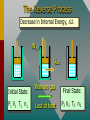
Thermodynamics THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating A THERMODYNAMIC SYSTEM • A system is a closed environment in which heat transfer can take place. (For example, the gas, walls, and cylinder of an automobile engine.) Work done on gas or work done by gas INTERNAL ENERGY OF SYSTEM • The internal energy U of a system is the total of all kinds of energy possessed by the particles that make up the system. Usually the internal energy consists of the sum of the potential and kinetic energies of the working gas molecules. TWO WAYS TO INCREASE THE INTERNAL ENERGY, U. +U WORK DONE ON A GAS (Positive) HEAT PUT INTO A SYSTEM (Positive) TWO WAYS TO DECREASE THE INTERNAL ENERGY, U. Wout Qout -U Decrease hot WORK DONE BY EXPANDING GAS: W is positive hot HEAT LEAVES A SYSTEM Q is negative THERMODYNAMIC PROCESS Increase in Internal Energy, U. Wout Qin Initial State: P1 V1 T1 n1 Heat input Final State: Work by gas P2 V2 T2 n2 The Reverse Process Decrease in Internal Energy, U. Win Qout Initial State: P1 V1 T1 n1 Work on gas Loss of heat Final State: P2 V2 T2 n2 THE FIRST LAW OF THERMODYAMICS: • The net heat put into a system is equal to the change in internal energy of the system plus the work done BY the system. Q = U + W final - initial) • Conversely, the work done ON a system is equal to the change in internal energy plus the heat lost in the process. SIGN CONVENTIONS FOR FIRST LAW • Heat Q input is positive +Wout +Qin U • Work BY a gas is positive -Win U • Work ON a gas is negative • Heat OUT is negative Q = U + W -Qout final - initial) APPLICATION OF FIRST LAW OF THERMODYNAMICS Example 1: In the figure, the Wout =120 J gas absorbs 400 J of heat and at the same time does 120 J of work on the piston. What is the change in internal energy of the system? Qin Apply First Law: Q = U + W 400 J Example 1 (Cont.): Apply First Law Q is positive: +400 J (Heat IN) Wout =120 J W is positive: +120 J (Work OUT) Q = U + W U = Q - W Qin 400 J U = Q - W = (+400 J) - (+120 J) = +280 J U = +280 J Example 1 (Cont.): Apply First Law Energy is conserved: The 400 J of input thermal energy is used to perform 120 J of external work, increasing the internal energy of the system by 280 J The increase in internal energy is: Wout =120 J Qin 400 J U = +280 J FOUR THERMODYNAMIC PROCESSES: • Isochoric Process: V = 0, W = 0 • Isobaric Process: P = 0 • Isothermal Process: T = 0, U = 0 • Adiabatic Process: Q = 0 Q = U + W ISOCHORIC PROCESS: CONSTANT VOLUME, V = 0, W = 0 0 Q = U + W so that Q = U QIN +U QOUT No Work Done -U HEAT IN = INCREASE IN INTERNAL ENERGY HEAT OUT = DECREASE IN INTERNAL ENERGY ISOCHORIC EXAMPLE: No Change in volume: P2 B P1 A PA TA = PB TB V1= V2 400 J Heat input increases P with const. V 400 J heat input increases internal energy by 400 J and zero work is done. ISOBARIC PROCESS: CONSTANT PRESSURE, P = 0 Q = U + W But W = P V QIN QOUT Work Out +U -U Work In HEAT IN = Wout + INCREASE IN INTERNAL ENERGY HEAT OUT = Win + DECREASE IN INTERNAL ENERGY ISOBARIC EXAMPLE (Constant Pressure): P A B VA TA 400 J Heat input increases V with const. P V1 = VB TB V2 400 J heat does 120 J of work, increasing the internal energy by 280 J. ISOBARIC WORK P A B VA TA 400 J V1 V2 = TB PA = PB Work = Area under PV curve W orkPV VB ISOTHERMAL PROCESS: CONST. TEMPERATURE, T = 0, U = 0 Q = U + W AND QIN U = 0 Q = W QOUT Work Out U = 0 Work In NET HEAT INPUT = WORK OUTPUT WORK INPUT = NET HEAT OUT ISOTHERMAL EXAMPLE (Constant T): PA A B PB U = T = 0 PAVA = PBVB V2 V1 Slow compression at constant temperature: ----- No change in U. ISOTHERMAL EXPANSION (Constant T): PA A B PB U = T = 0 VA VB 400 J of energy is absorbed by gas as 400 J of work is done by gas. T = U = 0 PAVA = PBVB TA = TB Isothermal Work VB W nRT ln VA ADIABATIC PROCESS: NO HEAT EXCHANGE, Q = 0 Q = U + W ; W = -U or U = -W U = -W W = -U U Work Out Q = 0 +U Work In Work done at EXPENSE of internal energy INPUT Work INCREASES internal energy ADIABATIC EXAMPLE: PA A B PB V1 Insulated Walls: Q = 0 V2 Expanding gas does work with zero heat loss. Work = -U ADIABATIC EXPANSION: PA A B PB Q = 0 PAVA TA VA 400 J of WORK is done, DECREASING the internal energy by 400 J: Net heat exchange is ZERO. Q = 0 = PBVB TB VB P AV A P BV B ADIABATIC EXPANSION/COMPRESSION According to the 1st Law of Thermodynamics: ΔU = Q – Wby but Q = 0, since walls are insulated ΔU = – Wby For a monatomic ideal gas: Wby U 32 nRTi T f The red curve shows an adiabatic expansion of an ideal gas. The blue curves are isotherms at Ti and Tf. Adiabatic curves can be approximated as linear. To Be Continued…