* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit B: Understanding Energy Conversion Technologies
Vapor-compression refrigeration wikipedia , lookup
Thermal conductivity wikipedia , lookup
Underfloor heating wikipedia , lookup
Thermal comfort wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Passive solar building design wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
Thermoregulation wikipedia , lookup
Solar water heating wikipedia , lookup
Insulated glazing wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
Cogeneration wikipedia , lookup
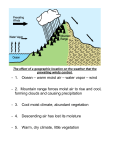
Dynamic insulation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Building insulation materials wikipedia , lookup
Intercooler wikipedia , lookup
Solar air conditioning wikipedia , lookup
Thermal conduction wikipedia , lookup
Unit B: Understanding Energy Conversion Technologies Science 14 Name: ___________________________________ Science 14 Name: ____________________________ Date: _________________________ •Introduction Energy Transfer – the transfer of energy from one body to another Terminology Heat Energy vs. Thermal Energy These two terms are essentially the same thing. They can be used interchangeably. Important Questions What causes heat? Where does it come from and why? For over a century, scientists hotly debated the answers to these questions as they worked to develop modern heat theory. 1 Science 14 Name: ____________________________ Date: _________________________ In a pre-match society, starting a fire was not the simple task it is today. In many early societies, people who could start fires easily were admired, often revered. If it was your job to keep the fire going, letting it go out was considered a grievous wrong. Friction Theory When two surfaces are rubbed together, the parts that touch resist movement This resistance is friction. Count Rumford used observations about friction to change the way scientists look at heat. Benjamin Thomson was an engineer and scientist who lived in the Thirteen Colonies at the time of the American Revolution. Because he stayed loyal to Britain, many Americans considered him a traitor. British loyalists, however, considered him a hero. Thomson was given the title of Count Rumford to reward him for his loyalty to Britain. 2 Science 14 Name: ____________________________ Date: _________________________ Rumford was an engineer/scientist who was hired to manufacture cannons in Munich, Germany. During this project, one worker carelessly touched a rod being used to bore a hold through a piece of metal. His hand was seriously burned. In the late 1700s, Count Rumford observed that heat was created when metal cut metal. This heat results from friction! Have you ever watched popcorn popping in an air popper? The random, dancing motion of the kernels is easy to observe. But what causes this motion? A similar type of motion is occurring in a glass of water. Robert Brown – ____________________________________________ During the 1800s he was using a microscope to observe ____________________ in a drop of ___________________. He noticed that although the microscope was quite still, the pollen grains ______________________________. When he _____________________ the temperature of the water, the motion ________________________. This motion has become known as _______________________________. 3 Science 14 Name: ____________________________ Date: _________________________ Making the observation was easy. But how can we explain it? At first, Brown thought that the pollen grains were _______________. Later he reasoned that water must be composed of tiny unseen _______________. These particles are in _______________, ________________ motion. The motion of the pollen grains must be caused by ___________________ between the ____________________ and the other unseen ______________. (Brown was unsure of what the particles were) Later, his evidence helped develop the _________________________________. The concept of heat is commonplace; what causes heat is fairly abstract. Technically, heat is defined as the ______________________________________ ____________ and is identified by a difference in _________________________. An object does not possess heat. Rather, an object possesses _________________ and can lose that energy in the process of _____________________. It is a common misconception to think of heat as a ____________ rather than a _______________. This probably stems from the original _________________ definition of heat. Scientists thought that something _______________________ left an object when it got colder. Recall that Rumford was able to gain a scientific understanding of heat through _____________________________________. Before Rumford, scientists thought heat was a ____________________________ ________________________. Rumford did not replace the ____________________. Rather, he demonstrated an __________________________ in the theory. Two later British scientists — Sir Humphry Davy and James Prescott Joule took Rumford’s ideas to the next step. 4 Science 14 Name: ____________________________ Date: _________________________ Davy, an English chemist, designed demonstrations to ___________________________ _______________________________. He rubbed __________ and other _________________ with low melting points together to show that they would melt with _________________________________. Joule determined the ___________________________________ of heat by measuring the ___________________________________ produced by friction. Working in imperial measure, he found that, on average, a weight of ________________ falling through a distance of one foot would raise the temperature of one pound of water by ______________. In addition, Joule’s investigations showed that heat is produced by _________________, contradicting the caloric theory. We can relate this to the calorie, which is related to _____________________. The calorie is a ___________________________. One calorie is the amount of energy needed to raise _______________ of water by 1°C. Other units of energy are the _____________ — named after James Prescott Joule and the British Thermal Unit (BTU). The BTU is used in Canada to rate ________________________ of stoves, ovens, and barbecues. 5 Science 14 Name: ____________________________ Date: _________________________ •Mysterious Motion: Summary: Practice! Check Your Understanding p. 85 6 Science 14 Name: ____________________________ Date: _________________________ •5.2 Heat and Temperature The modern theory of heat began with ___________________________. He was the first to suggest that the energy that came with heat – ___________________ – was related to the motion of unseen particles of a substance. But, what are these unseen particles? What causes the motion? Recall the Particle Theory of Matter: _____________________________________________________________ _____________________________________________________________ Kinetic – ____________________________ Kinetic Art – __________________ (i.e. Mobiles) Kinetic Energy – ______________________________ It is a measure of the amount of ___________________ particles have. We can use kinetic energy to explain the difference between _____________ and __________________________. A molecule can have three different forms of movement: __________________________________________________________ __________________________________________________________ __________________________________________________________ 7 Science 14 Name: ____________________________ Date: _________________________ The motion of particles can be compared to bumper cars! Like bumper cars, atoms and molecules ______________ with each other at different __________________. All particles have different _____________________________. So what is the difference between heat and temperature? Temperature – ____________________________________________________ Heat – ___________________________________________________________ Example: 8 Science 14 Name: ____________________________ Date: _________________________ Because temperature is a measure of ________________________ molecules are, and because molecules ____________ as they get colder, the coldest possible temperature is finite! Particles truly stop moving at ___________________. Absolute zero is ______________________. The lowest artificial temperature achieved to date is 0.003°K. The highest is estimated at 100 000 000°K and was from a nuclear blast. There are more temperature scales than Celsius and Fahrenheit. The _____________________________ starts at absolute zero and rises in degrees equal in magnitude to ________________ degrees. Other scales include the __________________ scale and the ___________________ ____________________________. Practice! Check Your Understanding p. 87 9 Science 14 Name: ____________________________ Date: _________________________ •5.3 Transfer of Heat There have been many predictions about when Earth will end. Fear of Y2K computer problems brought excitement to New Year’s Eve 2000. As we study heat transfer, we will learn about another proposed end to the universe Forms of Heat Transfer Heat flows from __________ to __________. The flow continues until both objects are at the same ______________________. But what is really happening? How does thermal energy transfer from one object to another? __________________________ Molecules placed on a hot burner will vibrate __________________. They have more ________________________ than the molecules in a cooler location (a cool pot). Contact between the pot and the burner causes the molecules of the hot burner to _________________ with the slower molecules of the cool pot. These collisions result in a transfer of _______________________. The molecules of the cooler pot start to ____________________, gaining kinetic energy. The molecules of the hot burner _______________, losing kinetic energy. This transfer of heat by contact is called ______________________. 10 Science 14 Name: ____________________________ Date: _________________________ Thermal conductivity and electrical conductivity are __________________. Substances that conduct electricity will also tend to be good ________________________________. _________________ is a good example of this. Conversely, __________ and _____________ are poor thermal and poor electrical conductors. ____________________________ If you hold your hand above a hot burner of a stove, you will feel a warm _______________________________. Why? Heat is transferred, by ___________________, from the hot burner to the air molecules touching the burner. These air molecules gain ___________________, vibrate faster, and get farther _________________. The warmer molecules are ____________________ than the cool air molecules, so the warm air is ______________________ than the cool air around it. This causes the warm air to ___________, creating the warm current that we feel. Cool, denser air rushes to take the place of the ___________________. Because of continuous air flow, all the air in the room will become ________________. This transfer of heat by movement is called ____________________. 11 Science 14 Name: ____________________________ Date: _________________________ ______________________________ Picturing a hand that is close to the side of a burner, but not above the burner can help us explain radiation. The front of the hand is not being heated by __________________ or ____________________. There is no __________________ with the burner and convection currents of warm air would rise _____________ from the hand. The front the hand is being heated by ______________________. Radiation is produced by _________________________________, which are tiny particles present in all atoms. This vibration makes a wave called an _________________________________ or ________________________________ wave. These waves are similar to the waves your hand can create when it vibrates in _________________________________. _____________ or _____________ run away from your moving hand. ________________________ waves travel from the burner. They strike the hand and transfer __________________________ to the molecules in the hand. This causes molecules in the hand to vibrate ________________. On a sunny day, most of the heat that you feel is the result of infrared radiation from the __________. Infrared radiation is one form of electromagnetic radiation. Other forms include ___________________, radio waves, visible light waves, and ______________. 12 Science 14 Name: ____________________________ Date: _________________________ Electromagnetic radiation travels in ________________. Each type of electromagnetic radiation has a specific ____________________ and moves through the vacuum of space. Summary Did You Know? Hot objects warm cool ones until their temperatures are the same. Some people believe this will happen to the __________________. According to the theory, the temperature of the entire universe will ___________ _____________________________________. When this happens, heat will no longer __________________________. Without a source of energy, life will be ______________________! This prediction is called the “_____________________________________.” 13 Science 14 Name: ____________________________ Date: _________________________ Practice! Check Your Understanding p. 91 •5.4 Heat Transfer in Nature ___________________________ causes many weather phenomena, such as winds. Many cottages are on the shores of lakes, where convection currents keep the cottages cool in the daytime and warmer at night. In the evening, the air __________ the water cools more ________________ than the air over the land. Warm air from over the water ____________ and moves toward the land, keeping it warm. During the day, cool air from the lake moves _______________ the warm land. Sea and Land Breezes Recall: ________________________, and ________________________. The circular movement that results is called ___________________________. All winds start with convection currents. Land and sea breezes are convection currents of air that occur near a shoreline. They are both created by _____________________________ near the surface of the Earth. 14 Science 14 Name: ____________________________ Date: _________________________ On a sunny day, _________________________ from the Sun strikes Earth’s surface. Heat is absorbed by _________ and _____________. But land and water heat at different _____________. Land heats ______________, but also cools quickly. Water heats _______________ and takes longer to cool. 15 Science 14 Name: ____________________________ Date: _________________________ How Oceans Help to Moderate Climates Oceans are capable of storing large amounts of _________________________. This prevents the area around them from having extreme ___________________ _____________. Oceans _________________ the climate of land areas near them. This means they prevent the area from becoming _______________ or ________________. But how??? In ____________________________, an ocean can release great amounts of heat without cooling much itself. Even during very hot or very cold days, and as seasons change, the temperature of oceans remains ____________________. As the sun warms the air above the ocean, heat flows from the air into the water, __________________________. When the temperature of the air above the ocean ____________, heat flows from ocean to air and ____________ the air. We could say that oceans prevent land near them from getting _______________ or _________________. Because of this, ______________________ (like Vancouver) have moderate climates. 16 Science 14 Name: ____________________________ Date: _________________________ Practice! Check Your Understanding p. 97 Heat Transfer Review (worksheet) Midpoint Review (worksheet) 17 Science 14 Name: ____________________________ Date: _________________________ •5.5 Heat Transfer and Technologies Many household technologies either _______________ or prevent the transfer of heat through _________________, _________________ or __________________. That is why metal cooking pots have hard plastic or wooden handles. What other techniques are used to handle hot food? _____________________ Most cooks want to control how food is _________________. Consider the following: heating soup on a stove top. The pot is heated by _____________________ through contact with the burner. The soup at the bottom of the pot _______________________ through conduction. Heated soup rises to the ___________, because it is ___________________. Colder soup near the top of the pot flows to the _____________ because it is _______________. This causes a circular motion (aka ___________________________) and allows the entire pot of soup to heat to a _____________________________. _____________________ When an oven is turned on, ____________________________ move heat inside the oven. Air at the bottom of the oven is heated by ___________________ or an ________________________________. The heated air becomes __________________ and _____________________ of the oven. The cooler air ___________________________. 18 Science 14 Name: ____________________________ Date: _________________________ The hot air heats the oven walls. These walls then radiate heat in all directions. Food in an oven then becomes cooked by both convection and radiation. There is also conduction if a baking pan is used. Did you know? ______________________ surfaces reflect more heat than __________ surfaces. This is why people in ____________________ often wear light-coloured clothing. Getting Rid of the Heat Combustion of fuel inside an engine produces a large quantity of __________________________. If this energy were not removed, the engine would ______________ and be damaged. How is it protected? 19 Science 14 Name: ____________________________ Date: _________________________ The engine’s cooling system contains a ____________________ (most likely ___________________). The coolant is pumped through the engine block to the __________________. A radiator is a honeycomb made of a ____________________. The metal alloy is a good ___________________. Heat from the coolant is _____________________ through this alloy to air. Either a _________ or the __________________________ forces this air through the radiator. Heat is transferred to the __________ that rushes through the radiator. These three techniques use ______________________ to protect engines from heat damage. Keeping it Cool When you put a little water on the back of your hand, your hand becomes ___________. That is because ________________________ transfers from your hand to the water through _________________________. Water absorbs heat from your hand and ________________________. Evaporation removes ________________________ as the water molecules leave the water droplets and move into the ___________. Your hand feels _______________. 20 Science 14 Name: ____________________________ Date: _________________________ This form of cooling is usually called __________________________. The evaporation caused the _______________, but the cooling action started with _________________ of heat _________ from your hand. Heat Transfer in a Refrigerator A. A fluid called a ________________ circulates through the pipes. B. Heat from the food transfers to the ___________________ surrounding it. _____________________ then transfers from the air to the coolant. C. The coolant evaporates as it gets ________________. It is pumped to the _________________. D. When it reaches the ___________________, pressure is applied to change it back into a ________________. E. The _____________________ is pumped to these coils. Thermal energy is __________________ into the room. The cycle starts again. 21 Science 14 Name: ____________________________ Date: _________________________ Air conditioners work in a similar manner. In this case, the back of the unit is _________________ the room or house. To keep the inside of the room cool, heat is dispersed to the _______________________. Although useful, coolant technology has an environmental cost. For the past 50 years, the liquid coolant used in refrigerators and air conditioners has been liquid chlorofluorocarbons, or CFCs. CFCs are responsible for atmospheric ozone depletion. As of January 2000, worldwide production of the most dangerous CFCs was to be replaced by alternative liquid coolants. Research is underway to produce environmentally safer but effective coolants. Practice! Check Your Understanding p. 101 REVIEW Chapter 5 Review p. 102 #s 1 – 12 Heat Transfer Crossword 22 Science 14 Name: ____________________________ Date: _________________________ •Chapter 6: Controlling Heat Transfer •Introduction Fire-walkers amaze tourists. They aim to convince their audiences that they have some sort of _________________________________. But! Fire-walkers have learned to reduce the transfer of __________________________ from the red-hot coals to their feet. But how is this done? *Hint: Cooks test to see if a skillet is hot enough by dropping water into it. The pan is hot enough if the water drops ________________across the surface of the skillet. We will re-visit the secrets of fire-walking later. We control heat transfer very day. Knowledge of differences in _________________________________ is used in many ways. •6.1 Absorbing and Losing Heat Recall from Chapter 5: Water moderates the __________________________ of the land around it. In this chapter we will discuss another reason why that happens. Different materials absorb heat at ____________________________. This is referred to as _______________________________. 23 Science 14 Name: ____________________________ Date: _________________________ Specific Heat Capacity Different substances require different amounts of _______________________ to raise their temperature the ________________________. This is true for all substances. Each substance requires a _____________________ amount of heat gain or loss to change its _________________________. Specific heat capacity – _____________________________________________ ________________________________________________________________ Measured in joules per gram degrees Celsius (i.e. ___________ or ___________) The specific heat capacity of a substance can change depending on its ____________. For example, solid water (ice) has a ____________ specific heat capacity than liquid water. This has to do in part with the _________________________ between the molecules. Molecules that are close together, as in a solid, have a stronger attraction to each other. They require more energy to get moving. The joule is named after ______________________________ (1818–1899), a brewer and physicist. It is represented by the symbol ________. Example The specific heat capacity for water is ________________ What does this mean? ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ Regardless of the amount of water, the amount of heat needed to change the temperature _________________________________. 24 Science 14 Name: ____________________________ Date: _________________________ Another example: The specific heat capacity of sand is ________________ This means: ______________________________________________________ ______________________________________________________ Consider: SHC of water = ______________ SHC of sand = ______________ Which has a higher specific heat capacity? This means that it takes more energy to increase the temperature of __________________ than it does to increase the temperature of _____________. This is why sand on a sunny beach is much warmer than the shallow water nearby. Common SHCs Substance Water Motor oil Vegetable oil Air glass sand Iron copper Water Motor oil Specific Heat Capacity 25 Science 14 Name: ____________________________ Date: _________________________ Warming Up and Cooling Down with Oceans Recall: oceans ______________________ shore areas Also, water has a ___________ specific heat capacity. Oceans store more heat energy or thermal energy than you might expect. With water’s large SHC, water can ______________, ______________ or _______________ much more thermal energy than land. That is another reason for land ______________ and ______________ quicker than lakes and oceans. SHC also affects climate in other ways: On a hot day, water will absorb heat. This slows down the rise of temperature in the _____________________________. At night, water will release __________. This slows cooling in the surrounding area. The secrets of fire-walking Water has a very high specific heat capacity (4.184 kJ/K kg), whereas coals have a very low one. Therefore the foot's temperature tends to change ___________ than the coal's. Water also has a high __________________________________, and on top of that, the rich blood flow in the foot will carry ___________ the heat and spread it. On the other hand, coal has a poor _________________________________, so the hotter body consists only of the parts of the coal which is close to the foot. When the coal cools down, its temperature sinks below the flash point, so it stops burning, and no _____________________ is generated. Firewalkers do not spend very much __________ on the coals, and they keep _________________. Calluses on the feet may offer an additional level of protection, even if only from pain; however, most people do not have calluses that would make any significant difference. 26 Science 14 Name: ____________________________ Date: _________________________ Practice! Check Your Understanding p. 110 •6.2 Keeping Heat at Home Recall: heat moves from ___________ toward ___________. This happens especially in the winter. Heat often leaks through ________________, _____________, and ______________. Proper ___________________ can help this problem. Insulation slows _______________________. With energy costs rising, Canadians want to make sure heat stays inside the house in winter. For Canadians, _________ to_________ percent of energy costs go toward heating or cooling our homes. If insulation is inadequate, much energy is _________________. This is bad for the environment and for the _________________________. 27 Science 14 Name: ____________________________ Date: _________________________ With insulation you get two benefits for the price of one. The same insulation that keeps heat ________during the winter keeps heat ______on a hot summer day. Knowledge of heat transfer teaches you how to keep your home _________ in the winter and __________ in the summer. But what makes a good insulator? Insulation reduces _____________________. A good insulator is the opposite of a good ___________________. With good insulation, the three forms of heat transfer are _____________. Heat convection and heat conduction can be minimized in two ways: First, create a _________________________ between the areas to be insulated. This is done in vacuum bottles and in some double-glazed windows with an inert gas between the panes. Second, use ____________________. Still air provides 15 000 times better insulation than metal. Substances used for insulation are chosen for their low ___________________________ and their ability to _____________________. Good conductors include __________, felt, cotton batting, ________________________, and spun glass or ______________________. ______________________ is an excellent insulator but it is a health hazard and no longer used in construction. Insulators work best when the air is _________. Moist air acts as a much better heat conductor than dry air. To keep insulation dry, fibreglass is covered with ____________________________ before drywall is installed. Without the plastic sheet, moisture from inside the house — from cooking, bathing, and breathing — would infiltrate the _________________ and reduce its ________________________. Radiant heat loss can be lessened by installing _______________________, _____________________________________, and ___________________________. 28 Science 14 Name: ____________________________ Date: _________________________ R-value Air transfers heat when it is moved by __________________________________. Air is an excellent insulator when it is held ______________. ________________ is a measure of how well an insulating material slows ________________________. Materials with high R-values are ________________ insulators than those with low R-values. i.e.) _____________________________________________________________ When materials are used together, the total R-value is the sum of the R-values of each material used. Example Walls in your home have 25 mm of expanded polystyrene and 25 mm of rigid urethane foam. What is the R-value of the insulation? ______________________________________________ Common R-Values Thickness of insulating material 25 mm of air space in a wall cavity 25 mm of air space with reflective surface on inside of wall cavity 25 mm of expanded polystyrene 25 mm of rigid urethane foam 25 mm of fibreglass 25 mm of solid wood 25 mm of wood shavings 25 mm of clay brick 25 mm of concrete One thickness of glass Thermal glass (2 thicknesses with air space) Approximate R-Value 29 Science 14 Name: ____________________________ Date: _________________________ Other Ways to Keep the Heat in Your House The empty space between the inside and outside wall of a house is called a ______________________. Filling the cavity with insulation stops _________________________________. When insulation fills a wall cavity, there are many pickets of trapped, still _____. _________________________ and ________________________ work in this way. Windows and Doors That Keep Heat In Windows and doors are two ______________________ when you try to keep a house warm. A single pane of glass is a poor _________________________. Heat escapes quickly through _____________. Leaks also develop around the panes and around the edge of the _____________. Older houses use double windows and doors – called ______________________ and ______________________ – to keep heat in. Today’s exterior doors and windows use ___________________________. This provides a space of ____________________. Insulation value of this still air in improved when air is mixed with a gas such as ___________________. Exterior doors used to be ________________________. Modern doors are cavities filled with insulation. Some are _________________ covered. To prevent heat transfer, there is a _______________ in the metal between the inside and outside. 30 Science 14 Name: ____________________________ Date: _________________________ Did You Know? Even the smallest crack (_________________) around the outside of one window, your furnace may burn an extra _______________ of fuel per day! Controlling Heat Transfer Pizza parlours keep pizza warm by transporting it in _______________________. As well as limiting heat transfer, the envelope must be __________________. This limitation prevents the use of some materials. A _________________________ uses several of the same technologies that keep your house warm in order to keep food and beverages warm. Inside is a ______________________________. One jar is fitted inside the other – similar to windows with a double pane. Some air is ___________________ from between the two jars. That is where the name comes from. The space between is a ________________________. __________________ or __________________ the glass away from the outer case. The cap is ____________________. 31 Science 14 Name: ____________________________ Date: _________________________ Kitchen and Workshop Large appliances (i.e. _______________________________________________) are all insulated. Insulation slows heat transfer, keeping ovens ________ and freezers _________. What makes a good insulator? ______________________ (i.e. Wood and plastic) Examples: ________________________________________________ ________________________________________________ Practice! Check Your Understanding p. 119 32 Science 14 Name: ____________________________ Date: _________________________ ●6.3 Keeping Yourself Warm The same techniques that keep our houses warm, keeps our _______________ warm. For example, on a cold day we may choose to wear several layers. We choose inner layers for their __________________ and _________________. Air trapped in the material serves as _______________________. A windproof outer layer keeps warm air from ___________________. Clothing insulates by ________________ air between fibres. The better clothes trap air, the better __________________ they are. This is why thicker clothing tends to be _______________ than thinner. Thicker clothing ________________ air flow and maintains _________________. Windproof material _____________ or ________________ airflow through the fibres. Some of the warmest winter clothing contain ___________. ___________ grow fluffy, down feathers. These feathers keep birds ___________. Down is often _____________ between the outer shell and inner lining of a vest or jacket. When down is ___________, it holds air in place. The jacket material keeps air from blowing through the down and changing _____________ for ______________. Recall: a dry body is a __________ body. Vigorous activity causes sweat. Water causes ________________ as it evaporates. When working or playing outside, you want to ____________ yourself from getting and staying ____________. When you start to sweat, _________________________________, possibly another. This allows heat to _____________________ and your body to stay at the right __________________________. When you stop moving you can _____________________ those layers. 33 Science 14 Name: ____________________________ Date: _________________________ People of the North Inuit designed clothing is the ___________________. Traditionally, Caribou Inuit clothes have two _________________________ on cold days. The inner set is worn with fur next to the __________. Body moisture is transferred through the _______ and through the __________________. Caribou fur is ______________; individual hairs are ______________. Air trapped between and inside the hair provides ________________________. The outer parka is worn with the fur _________________ – this is important for very ___________________. The parka hood traps air __________________ of the wearer’s face. Frigid air is __________________ before being breathed. This protects the wearer’s _________________. During extremely cold weather, water vapour from the lungs can ________________ and freeze on people’s ___________ and _______________. The hoods are designed to prevent ________________________. The edge of the hood, where ice might form, is trimmed with __________. Ice does not stick to __________________, __________, or some _________ fur. ________________ and ________________ Inuit wear up to four layers on their feet in winter. ___________________ is preferred to boots because it is __________________. Inuit parkas are much larger than we might expect. The large size allows wearers to bring their ________________ this warm space. 34 Science 14 Name: ____________________________ Date: _________________________ Did You Know? Some motorcyclists have clothing with built in _______________! Heating elements are sewn into the clothing and warm areas of the body such as the ______________ where a lot of heat can be lost. The clothing runs off of a motorcycle ________________ and uses less power than a headlight. The amount of heat can be controlled using a palm-sized ________________. Did You Know? People outside in winter protect themselves from frostbite. Frostbite usually freezes __________, ___________ and the _______________. One way to warm a cold hand is to tuck it into your ___________________ for a few minutes! 35 Science 14 Name: ____________________________ Date: _________________________ Keeping Cool Ever notice that people who live in warmer climate areas where more clothes? This is because ____________________________________________________. They wear _________, _________ robes to protect the body from the Sun’s ______________. These clothes are usually _____________ in colour to help ___________ heat and to allow body heat to _____________. Oven mitts work in a similar manner. Their quilted material __________________ heat transfer. Some include _______________________ material that also reduces heat transfer. Dressing for Intense Heat – or Cold __________________________ and _______________________ have to deal with major changes in temperature. But how? 36 Science 14 Name: ____________________________ Date: _________________________ Firefighters’ suits are made of a special material. Many contain __________________________ chemicals. When flames or sparks come into contact with the suit, the fabric ____________ but does not _____________. The charred material produces a layer of ______________________ that protects the firefighter from too much heat. Firefighters can suffer from ______________________ if their body temperature increases too much. Material on the inside of their fire suit __________________ body moisture. This helps to keep them ____________. Firefighters must monitor their own bodies. If they get too hot, they could suffer from heatstroke – even if it’s in the _____________! The fabrics used in firefighters’ suits are tested to determine their ___________________ qualities. They are tested by Scientists to see if they are ______________________ for firefighters to wear. This is done by dressing a __________________ in fire gear and setting it on _________. These mannequins are equipped with ________________ to measure the __________ and ______________ of heat transfer from the fire. Even in hot climates, temperatures deep underwater can be as cold as _______________. As a result, a diver’s suit should fit ________________. Tight diving suits prevent ____________________ next to the skin from causing cooling by ___________________. If ______________________________ moved in and out of the suit, a diver would soon be cold. Water could pick up heat from the diver’s body and carry it off into the ______________. 37 Science 14 Name: ____________________________ Date: _________________________ Dive suits are made of ________________ that has bubbles of ______________ trapped in the fabric. The more gas trapped in the fabric, the _______________ thermal value. These neoprene suits are well ________________ to keep body heat inside. Dive suits also have ___________ for the same reason that winter parkas have hoods. Underwater, a great amount of heat can be lost from a diver’s ___________. Some dive suits have ________________ added to the side of the fabric that is next to the skin. The shiny titanium _______________________ back into the body. Practice! Check Your Understanding p. 125 38