* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The African Eye Worm: A Case Report and
Survey
Document related concepts
Transcript
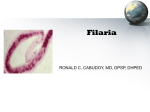
50 The African Eye Worm: A Case Report and Review Sadia Ali, MD,* Melanie Fisher, MD, MSc,* and Gregory Juckett, MD, MPH† *Section of Infectious Diseases and †Department of Family Medicine, West Virginia, Morgantown, WV, USA DOI: 10.1111/j.1708-8305.2007.00166.x Loiasis, caused by the filarial nematode Loa loa, is often asymptomatic but frequently manifests as episodic angioedema and periocular migration of adult worms. Hence also known as the eye worm.1 It is rarely encountered in the United States among travelers and immigrants. This report describes a case of loiasis in a Cameroonian student seen at a US university clinic. Case Report A 30-year-old African student from Kumba, Cameroon, was evaluated in a university travel clinic for a “foreign body sensation” in his right eye which lasted for 5 hours. At the time of these symptoms, he had visualized a thin, clear worm traveling across the right eye. After the worm had passed, the foreign body sensation resolved but was followed with intense conjunctivitis. His past history was significant for malaria 6 years ago but was otherwise unremarkable. He had been residing in the United States for 2 years during which time he had no history of international travel. He denied any migratory swellings or rash. On evaluation the following day, he had conjunctival injection of the right eye, but no worm was visualized on gross examination or on evaluation under a slit lamp. Physical examination was otherwise unremarkable. Additional investigations showed a white blood count of 6,400/L with 8% eosinophils (range: 1%–6%). Liver enzymes and renal functions were normal. Peripheral blood smear drawn at 3 pm was significant for the presence of sheathed microfilariae of Loa loa (Figure 1). The microfilariae were quantified at 4,910 microfilariae/mL of blood. Loa loa polymerase chain reaction was also positive, establishing the diagnosis of loiasis. Concomitant infection with onchocerca was ruled out by skin Corresponding Author: Sadia Ali, MD, Robert C. Byrd Health Sciences Center, Section of Infectious Diseases, West Virginia University, PO Box 9163, Morgantown, WV 26506-9163, USA. E-mail: [email protected] snips taken from bilateral shoulders, hips, and thighs. The patient was referred to the National Institute of Health for treatment and underwent apheresis on hospital days 1 and 2. Subsequently, he received prednisone 40 mg/d for 3 days which was rapidly tapered. Diethylcarbamazine (DEC) was then administered in incremental doses with 50 mg on day 1, followed by 50 mg three times per day for the next 3 days. The dose was gradually increased to 200 mg three times a day to complete 21 days of therapy. Microfilarial quantification at the time of discharge was 2,250 microfilariae/mL of blood. The treatment was tolerated well. On follow-up evaluation, no clinical relapse was detected and blood smear was negative for any microfilariae. The patient will be followed at 6-month intervals to monitor for relapse. Discussion Loiasis is endemic in the rain forests of Central and West Africa. Between 3 and 13 million people are estimated to be infected with it.2 The presence of three terminal nuclei and observation of sheathed microfilariae (approximately 290 by 7.5 m) in a daytime blood specimen are features characteristic of L loa. These characteristics help differentiate this organism from the blood-borne microfilariae of Wuchereria bancrofti and Mansonella perstans, whose geographic distribution overlap that of L loa.3 The microfilariae of M perstans are much smaller, have no sheath, and have nuclei extending to the end of the tail, whereas those of W bancrofti are sheathed microfilariae seen only on specimens drawn at night.4 © 2008 International Society of Travel Medicine, 1195-1982 Journal of Travel Medicine, Volume 15, Issue 1, 2008, 50–52 51 African Eye Worm Figure 1 Giemsa-stained peripheral smear from the patient showing the presence of sheathed microfilaria of Loa loa with nuclei extending to the tip of the tail. Humans acquire L loa from the day-biting female Chrysops fly, also known as the tabanid fly (horse fly or deer fly). The fly infects humans with the thirdstage filariform larvae which mature into adult worms over a period of 3 months to reside in the subcutaneous tissue. Within a year, the adult worms may produce thousands of immature larval microfilariae which can be detected in the blood stream. It is the circulating microfilariae which are responsible for transmission of infections after being taken up during the blood meal of female flies.3 Most people with L loa infection are asymptomatic. Disease manifestations are usually due to hypersensitivity reactions to the adult worms, and patients often present with Calabar swellings due to transient angioedema related to hypersensitivity reaction to the adult parasite migrating through subcutaneous tissue. The worm may also traverse the eye where it may remain visible as a thin transparent worm for hours before continuing its migration elsewhere. Adult worms may survive for 4 to 17 years. Intense itching, urticaria, and elevated eosinophil counts may be seen during the course of the illness.2 The most serious complication of L loa infection is meningoencephalitis, which may result as a potential consequence of high microfilarial load in the cerebrospinal fluid. Following treatment, dying microfilaria generate a host immune response when microfilaria levels exceed 2,500/mL, which can result in multiple cerebral infarcts, encephalitis, or cerebral edema.3 The treatment of L loa infection remains problematic despite recent advances made in anthelmintic chemotherapy. The current recommended drug of choice is DEC at a dose of 8 to 10 mg/kg/day for 21 days in gradual incremental doses. However, it is contraindicated in patients with elevated microfilariae levels because of its association with severe neurological side effects.5,6 Though DEC is thought to be curative in most cases, multiple courses of therapy may be needed and relapses may still occur. DEC has activity against both microfilariae and adult worms, and therefore, a sustained decrease in microfilarial intensity occurs following treatment.7,8 Mild side effects including fever, urticaria, and Calabar swellings which occur shortly after initiation of therapy generally respond well to corticosteroids or antihistamines. Albendazole has been shown to temporarily reduce microfilaremia when used for 3 weeks at doses of 200 mg twice a day; however, relapses are likely to occur.5,9,10 Concomitant L loa infection in patients with underlying Onchocerca volvulus infection is particularly problematic. Agents like ivermectin which were widely distributed throughout West Africa for the safe and effective control of onchocerciasis have been associated with severe post-treatment effects including death when administered to patients with concomitant L loa and O volvulus infection.5,11 Due to these adverse effects, the public health use of this drug in the forest habitat of the L loa vector has been constrained. Occurrence of serious reactions was related to the intensity of pretreatment L loa microfilaremia. The relative risk of developing marked or serious reactions was significantly higher when the L loa load exceeded 8,000 microfilariae/mL and are particularly notable in patients with L loa microfilaria greater than 20,000 to 50,000 microfilariae/mL.5,11 Since our patient was from an area endemic for both L loa and onchocerca, skin snip testing was done initially to rule out onchocerca. Apheresis was then performed to decrease the level of microfilaremia before administering escalating doses of DEC to minimize the possibility of side effects. This case illustrates how increasing travel has made it possible for such tropical diseases to be seen in nonendemic countries, usually in immigrants or travelers returning from west and central Africa. Knowledge regarding this disease entity is imperative to aid early recognition and to offer appropriate treatment by physicians worldwide. This patient was successfully treated and continues to remain symptom free to date. Acknowledgments The authors are grateful to Dr Patricia Canfield for providing the photograph and to Dr Amy Klion of the National Institutes of Health for all her help and guidance with this case. J Travel Med 2008; 15: 50–52 52 Declaration of Interests The authors state that they have no conflicts of interest. References 1. Nutman TM, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: Recognition of a hyper-responsive syndrome with characteristic clinical manifestations. J Infect Dis 1986; 154:10–18. 2. Klion A, Nutman TB. Loiasis and mansonella infections. In: Guerrant R, Walker DH, Weller PF, eds. Tropical infectious diseases: principles, pathogens and practice. Vol 2. Philadelphia, PA: Churchill Livingstone, 1999:861 3. Klion A. Loiasis. In: Strickland GT, ed. Hunter’s tropical medicine and emerging infectious diseases. 8th Ed. Philadelphia, PA: W.B. Saunders Company, 2000:754–756. 4. Grove DI. Tissue nematodes including Trichinosis, Dracunculiasis and Filariasis. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. Vol 2. 6th Ed. Philadelphia, PA: Churchill Livingstone, 2000:3267–3275. J Travel Med 2008; 15: 50–52 Ali et al. 5. Tabi TE, Befidi-Mengue R, Nutman TB, et al. Human loiasis in Cameroonian village: A double blind, placebo controlled, crossover clinical trial of a three day albendazole regimen. Am J Trop Med Hyg 2004; 71:211–215. 6. Carme B, Boulesteix J, Boutes H, Puruehnce MF. Five cases of encephalitis during treatment of loiasis with diethylcarbamazine. Am J Trop Med Hyg 1991; 44:684–690. 7. Ottsen EA. Efficacy of diethylcarbamazine in eradicating infection with lymphatic dwelling filariae in humans. Rev Infect Dis 1985; 7:34. 8. Wahl G, Geroges AJ. Current knowledge on the epidemiology, diagnosis, immunology, and treatment of loiasis. Trop Med Parasitol 1995; 46:287. 9. Klion AD, Horton J, Nutman TB. Albendazole therapy for loiasis refractory to diethylcarbamazine treatment. Clin Infect Dis 1999; 29:680–682. 10. Klion AD, Massougbodji A, Horton J, et al. Albendazole in human loiasis: Results of a double blind, placebo controlled trial. J Infect Dis 1993; 168: 202–206. 11. Gardon J, Gardon-Wendel N, Demanga N, et al. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997; 350:18–22.