* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Standard PDF - Wiley Online Library
Overexploitation wikipedia , lookup
Unified neutral theory of biodiversity wikipedia , lookup
Ecological fitting wikipedia , lookup
Island restoration wikipedia , lookup
Biological Dynamics of Forest Fragments Project wikipedia , lookup
Occupancy–abundance relationship wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
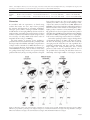
Oikos 124: 1391–1403, 2015 doi: 10.1111/oik.02222 © 2015 The Authors. Oikos © 2015 Nordic Society Oikos Subject Editor: Paulo Guimares Jr. Editor-in-Chief: Dries Bonte. Accepted 11 December 2014 Carcass size shapes the structure and functioning of an African scavenging assemblage Marcos Moleón, José A. Sánchez-Zapata, Esther Sebastián-González and Norman Owen-Smith M. Moleón ([email protected]) and N. Owen-Smith, School of Animal, Plant and Environmental Sciences, Univ. of the Witwatersrand, Wits 2050, Johannesburg, South Africa. – M. Moleón and J. A. Sánchez-Zapata, Depto de Biología Aplicada, Univ. Miguel Hernández, Ctra. Beniel km 3.2, ES-03312 Orihuela, Alicante, Spain. – E. Sebastián-González, Depto de Ecologia, Univ. de São Paulo, CEP 05508-900, São Paulo, Brazil. The particle size of the food resource strongly determines the structure and dynamics of food webs. However, the ecological implications of carcass size variation for scavenging networks structure and functioning have been largely overlooked. Here we investigate differences in scavenging patterns due to carcass size in a complex vertebrate scavenger community, Hluhluwe-iMfolozi Park, South Africa, while taking into account seasonality. We monitored the consumption of three types of experimental carcasses: ‘small’ ( 10 kg), ‘medium’ (10–100 kg) and ‘large’ ( 100 kg). We employed general lineal models to explore the influence of carcass size on 1) scavenging network structure (scavenger species richness per carcass) and 2) functioning (carcass detection time, consumption time, consumption rate and percentage of carrion consumed). We also tested whether the structure of the scavenging network of each carcass size was nested, i.e. whether the scavenging assemblage in species-poor carcasses was a subset of the assemblage consuming species-rich carcasses. We found strong evidence indicating that carcass size is a major factor governing the associated scavenger assemblage. Scavenger species richness per carcass and carcass consumption time and rate increased with carcass size, while carcass detection time and percentage of carrion biomass consumed were negatively related to carcass size. Strikingly, most of the carrion biomass was consumed by facultative scavengers, represented by large mammalian carnivores, rather than by obligate scavengers (i.e. vultures). Scavenging network nestedness tended to be higher at larger carcasses, and nestedness was sensitive to the removal of the most connected species in the network (spotted hyena) rather than vultures. When comparing scavenging and predation assemblages, crucial size-dependent differences emerge. Also, we identified a traditionally ignored mechanism by which hunting large prey could be relatively less profitable for predators, namely the costs associated with competition from scavengers and decomposers. The importance of scavenging in community and food web ecology and evolution has not been fully recognized until quite recently (DeVault et al. 2003, Wilmers et al. 2003, Selva et al. 2005, Selva and Fortuna 2007, Wilson and Wolkovich 2011, Beasley et al. 2012, Barton et al. 2013, Moleón et al. 2014a, b, Pereira et al. 2014). Neglecting the direct and indirect interactions involving carrion could lead to serious misinterpretations within food-web and energy flux models (Wilson and Wolkovich 2011, Moleón et al. 2014a). Understanding the factors that affect carcass use by scavenging animals therefore has wide ramifications for biodiversity conservation and management (DeVault et al. 2003, Wilson and Wolkovich 2011, Moleón et al. 2014a, b). Scavenging patterns may depend on features of the carcass (e.g. cause of death, spatio-temporal distribution), the consumer (e.g. body size, sociality, circadian activity) or extrinsic variables (e.g. weather conditions, availability of other food resources, micro-habitat structure; DeVault and Rhodes 2002, DeVault et al. 2003, 2004, Selva et al. 2003, 2005, Wilson and Wolkovich 2011, Kendall et al. 2012, Olson et al. 2012, Ruzicka and Conover 2012, Sebastián-González et al. 2013, Moleón et al. 2014a). However, scientific knowledge about the relative contribution of each influence is still very limited. One of the major factors that may govern scavenging patterns by vertebrates worldwide is carcass size (DeVault et al. 2003, Sebastián-González et al. 2013, Moleón et al. 2014a). Prey (in a wide sense) size is closely associated with the strength and direction of inter-specific interactions, which in turn determine the structure and dynamics of food webs and other ecological networks (Woodward et al. 2005). Prey size has been a matter of research for many years in fields such as the ecology of predation (Cohen et al. 1993, Sinclair et al. 2003, Owen-Smith and Mills 2008). However, although terrestrial vertebrate carcasses span a very wide size range, from a few grams (e.g. shrews) to several metric tons (e.g. rhinoceroses and elephants), hardly any study has explicitly explored the ecological implications of food particle size in a scavenging context (but see DeVault et al. 2004). Recently, Sebastián-González et al. (2013), who studied patterns of 1391 consumption of lagomorph carcasses, hypothesized that larger carcasses may be partitioned between more scavenger species, promoting facilitative and competitive processes that might eventually lead to more organized (e.g. nested) scavenging networks. A nested scavenging pattern arises when the assemblages of scavengers feeding on carcasses visited by few scavengers are subsets of the scavenger assemblages of carcasses visited by many scavengers (Selva and Fortuna 2007). This is supported by both theory (Moleón et al. 2014a) and findings of field studies that used ungulate carcasses, where inter-specific interactions among several carrion consumers were frequent (Cortés-Avizanda et al. 2012) and the scavenging pattern was indeed clearly nested (Selva and Fortuna 2007). This contrasts with the pattern identified for predator–prey relationships among large mammals, because smaller ungulates are killed by more species of carnivore than larger ones (Sinclair et al. 2003). Regarding scavenging efficiency, DeVault et al. (2004) found that the probability of carcass removal by vertebrates was higher for large than for small rodent carcasses. These authors suggested that this is because larger carcasses are more conspicuous (i.e. easier to find) and comparatively less exploited by decomposers due to a lower surface:volume ratio. These patterns need further empirical support taking into account wider variation in carcass size. Besides carcass size, the structure and functioning of scavenger communities can also vary between seasons. Most mammalian carnivores, as well as avian meso-carnivores such as raptors and corvids, are opportunistic or ‘facultative scavengers’, as they shift from hunting to scavenging depending on the opportunities presented (DeVault et al. 2003, Wilson and Wolkovich 2011, Moleón et al. 2014a, Pereira et al. 2014). Among terrestrial vertebrates, only the vultures are ‘obligate scavengers’, totally dependent on carrion as their food resource (Moleón et al. 2014a). Scavenging opportunities for facultative scavengers depend strongly on seasonal variation in prey susceptibility to mortality (Pereira et al. 2014). Seasonal shifts in access to carrion by facultative scavengers can change the scavenging rates of not only facultative scavengers (Wilmers et al. 2003, Selva et al. 2005), but also of vultures through a wide array of direct and indirect interactions (Moleón et al. 2014a). During the dry season, less vegetation cover might also favor the detection of carrion by scavengers, especially mammals (Ruzicka and Conover 2012). However, seasonal changes in scavenging patterns have largely been ignored in the scientific literature. The general goal of our study was to investigate differences in scavenging patterns due to carcass size in a complex African vertebrate scavenger community. We addressed three main questions: 1) is the scavenging network richer in species, and more nested, as carcass size increases? 2) Is consumption efficiency a function of carcass size? 3) Do the scavenging network and the consumption efficiency differ seasonally? We hypothesize that both the assemblage structure and functioning may be carcass size-dependent. Our predictions are that larger carcasses will be exploited by a greater number of scavenger species, and that the scavenging network exploiting larger carcasses will be more nested than that utilizing smaller carcasses. In turn, more species and more organized assemblages could lead to higher consumption rates. Regarding seasonality, we expect that carcasses are 1392 detected earlier during the dry season when compared with the wet season. Based on our findings, we discuss potential structural differences between scavenging and predation networks. Material and methods Study area Hluhluwe-iMfolozi Park (HiP hereafter) is located in the KwaZulu-Natal province of South Africa (28°00′–28°26′S, 31°43′–31°09′E). HiP is a ca 900 km2 wildlife reserve ranging from 60 to 750 m a.s.l., being hilly in the northern section (Hluhluwe) and flatter in the south (iMfolozi). Mean annual precipitation ranges from 985 mm in Hluhluwe to around 600 mm in parts of iMfolozi. The majority of rainfall occurs in the summer months, from October to March. Perennial surface water is irregularly available in the Hluhluwe River in the north, and the Black and White Umfolozi Rivers in the south. As a result of both topography and rainfall, there is a north-to-south contrast in vegetation, from grassland-forest mosaic with extensive thickets to thorn savannah (Grange et al. 2012). Long-distance movements of herbivores are prevented by boundary fences, and neither severe droughts nor disease outbreaks took place during our study period. Herbivore and scavenger assemblages HiP retains a rich assemblage of vertebrate herbivores and carnivores. Ungulates ranged in size from 10 kg steenbok Raphicerus campestris to rhinoceroses Ceratotherium simum and Diceros bicornis and elephants Loxodonta africana weighing several metric tons, but with the greatest numeric abundance represented by antelopes in the size range 50–500 kg. Large mammalian carnivores included spotted hyena Crocuta crocuta, lion Panthera leo, African wild dog Lycaon pictus, leopard P. pardus and cheetah Acynomix jubatus. The most frequent mammalian meso-carnivores and omnivores were large-spotted genet Genetta tigrina, various mongooses Ichneumia albicauda, Galerella sanguinea and Mungos mungo, and bushpig Potamochoerus larvatus. Reptilian carnivores included Nile crocodile Crocodylus niloticus and two monitor species Varanus albigularis and V. niloticus. At least 17 species of birds of prey (Family Accipitridae excluding vultures) and two corvids Corvus albus and C. albicollis are commonly seen in the reserve. Among vultures, the white-backed vulture Gyps africanus is relatively abundant, while the lappet-faced vulture Aegypius tracheliotos and white-headed vulture A. occipitalis are uncommon. The Cape vulture G. coprotheres, hooded vulture Necrosyrtes monachus and palm-nut vulture Gypohierax angolensis are present only occasionally (Howells et al. 2009–2013, Moleón unpubl.). Most of the scavenging birds were resident year-round, but yellow-billed kites Milvus aegyptius were present only during the wet season. Study design and data sampling Between 2010 and 2012, we investigated carcass consumption patterns by the vertebrate scavenger community of HiP. We provided three types of experimental carcasses differing categorically in size: ‘small’ ( 10 kg), ‘medium’ (10–100 kg) and ‘large’ ( 100 kg). For small carcasses, we used six-week old, dirty white feathered chickens weighing ca 2 kg (n 21). For medium-sized carcasses (n 12) we used either impalas Aepyceros melampus(weight: 40–55 kg, four adult males) or nyalas Tragelaphus angasi (weight: 40–120 kg; three adult males, three adult females, one juvenile male and one yearling female). Large carcasses (n 8) were constituted by blue wildebeest Connochaetes taurinus (weight: 290 kg; one adult male), African buffalo Syncerus caffer (weight: 354–650 kg; one adult male and two adults of unknown sex), white rhino (weight: 2000 kg; one adult male) and elephant (weight: 4000 kg; three adult males or part thereof ). Six additional chickens, one impala, two nyala, one buffalo and two wildebeest carcasses were not included in the analyses because of camera failure. Carcasses were located randomly across the reserve, except for five large carcasses left where the animals had died. Chickens were distributed over the same locations as larger carcasses, after an interval of 5 months. Most carcasses were intact when the camera was activated, except for six large carcasses. Five of these were monitored 10 h after scavengers had started to eat while the sixth consisted of two legs without skin of a poached elephant (weight: 600 kg). Carcasses were equitably distributed between seasons (Table 1), and always in sites with intermediate vegetation cover (Ruzicka and Conover 2012). All carcasses but the elephant and rhino carcasses (i.e. the heaviest ones) were fixed to the ground or a tree trunk by means of wires or chains to prevent scavengers from moving the carcass away from the camera focus. Plant material was used to hide the fixing devices. Carcasses were placed randomly in time between dawn and dusk. Carcasses where monitored using automatic cameras activated by movement. Cameras were located in discreet places close to the carcasses (5–10 m away), and the intervening area was cleaned of grass and shrubs in order to ensure good visibility for the pictures. Cameras were programmed to take one picture every two minutes, provided that there was some movement, and worked 24 h a day (see Blázquez et al. 2009 for further details). Cameras were collected seven days after their activation or following complete consumption of the carcass. We considered a carcass to be totally consumed when only parts of the skeleton (head and big bones) were left or if taken away from the camera focus by a large scavenger. In the last case, we only considered carcasses in which the part taken was small enough to be consumed completely by the scavenger, usually a hyena. From the pictures we calculated the following variables related to scavenger species diversity: ‘richness’ (number of scavenger species per carcass) and ‘total richness’ (total number of scavenger species in all carcasses). For some purposes, we grouped scavenger species as ‘obligate scavengers’ (OS; i.e. vultures) and ‘facultative scavengers’ (FS; avian, mammalian and reptilian carnivores). Facultative scavengers were further separated in ‘large facultative scavengers’ (LFS; i.e. lion, leopard, cheetah, hyena, wild dog and crocodile) and ‘meso facultative scavengers’ (MFS; all of the smaller carnivores). Furthermore, MFS were separated in ‘avian facultative scavengers’ (aFS) and ‘mammalian facultative scavengers’ (mFS; we did not use the category ‘reptilian facultative scavengers’ because reptiles – crocodiles – only appeared at one large carcass). Regarding scavenging efficiency, we estimated for each carcass: ‘carcass detection time’ (time elapsed since the carcass was available until the arrival of the first scavenger), ‘carcass consumption time’ (time elapsed between when the carcass became available and its complete consumption), ‘carcass consumption rate’ (carrion biomass consumed by scavengers divided by carcass consumption time), and ‘percentage of carrion consumed’ (percentage of the carcass, excluding the stomach content, consumed by the scavengers). The stomach content was estimated as 5% and 10% of the total carcass weight for chickens and ungulates (Selva 2004), respectively. Non-consumed parts were estimated visually from the camera pictures and inspections of the carcass sites at the moment of the camera inactivation as a proportion. We calculated the biomass consumed by each scavenger group using the carcass weight without the stomach content and nonconsumed parts, the information provided by the pictures, the maximum food intake of each scavenger species (Estes 1992, Donázar 1993) and the minimum number of different individuals of each species at the carcass. Initial carcass weight was 2 kg for chickens. For ungulates the body mass was obtained from the literature taking into account sex and age (Supplementary material Appendix 1 Table A1). Statistical analyses First of all, we constructed species richness curves with BiodiversityPro (James and McCulloch 1990) to assess whether our sample size was sufficient to detect all the species that scavenge on a given carcass type. Species richness analysis (Magurran 1991) randomly pools the samples and examines how specific indicators accumulate as the samples are pooled. We plotted a graph of the species richness (i.e. number of scavenger species) against the number of pooled samples (i.e. carcasses). The samples are pooled from left to right. When an asymptote is reached all the species that Table 1. Number of carcasses monitored and recorded pictures per carcass type. Small carcasses were placed during the winter–summer transition. Scavenger species richness recorded at each carcass type is also shown, measured as mean richness (mean number of scavenger species per carcass) and total richness (total number of scavenger species). Note that the sum of species found in all carcass types excludes redundant species. n Carcass size Small Medium Large Total Winter Summer Total 8 7 4 19 13 5 5 23 21 12 9 42 Mean no. of pictures ( SD) 16.62 15.51 67.00 54.28 469.13 372.48 Total no. of pictures 349 813 3753 4915 Mean richness ( SD) Total richness 1.95 1.12 2.83 1.40 4.75 1.83 14 9 11 18 1393 scavenge on a given carcass type are supposed to have been detected and no further sampling is required. Then we tested whether scavenger species richness was related to carcass size, season and section of HiP. To do so, we fitted generalized linear models (GLMs) where ‘richness’ was the response variable, and ‘carcass size’ (small, medium and large), ‘hour’ of carcass placement (morning – from dawn to 12:00 h – and afternoon – from 12:00 h to dusk), ‘season’ (wet and dry) and ‘section’ (Hluhluwe and iMfolozi) were the categorical predictors. Within each of the two sections of HiP, the management history, topography, precipitation, vegetation and faunal composition (including vertebrate herbivores, carnivores and vultures) is quite homogeneous (Brooks and Macdonald 1983, Grange et al. 2012). Thus, the variable ‘section’ probably captures the bulk of the spatial variation component of our data. We evaluated the complete set of models with four or less parameters (in order to guarantee at least 10 observations per parameter) that includes these three explanatory variables. We used Poisson error distributions and log link functions. Model selection was based on Akaike’s information criterion for small sample sizes (AICc). This approach allows the most parsimonious model (lowest AICc) to be identified and ranks the remaining models. Delta AICc (ΔAICc) was calculated as the difference in AICc between each model and the best model in the set. We considered models with ΔAICc 2 to have similar support (Burnham and Anderson 2002). Following Burnham and Anderson (2004), we computed the Akaike weights (AICcw) for the set of models (ΔAICc 2) to assess the weight of evidence in favor of each candidate model. To explore the overall degree of support for the candidate models we also calculated their proportion of explained deviance (D2) according to this formula: D2 (null deviance – residual deviance) / null deviance 100 (Burnham and Anderson 2002). We also explored the scavenging network structure separately for small, medium and large carcasses. In particular, we investigated whether the pattern of consumption of each carcass size by the scavenger community was nested. Given that scavenger species richness was only weakly related to season (Results), we combined data from the wet and dry seasons for this purpose. To analyze nestedness, we constructed a binary matrix for each carcass type where each column represents a different scavenger species and each row represents a different carcass. The matrix cells aij 1 when the scavenger i used the carcass j and zero otherwise. We used the ANINHADO software (Guimarães and Guimarães 2006), which uses a metric developed by Almeida-Neto et al. (2008) called NODF (acronym for nestedness metric based on overlap and decreasing fill). This metric has been identified to be good for any type of nestedness analysis (Ulrich et al. 2009). The metric tends to 100 for highly nested communities, while random matrices will show intermediate NODF values. As nestedness may arise from a random community, ANINHADO compares the NODF value of each assemblage with the NODF of 1000 matrices constructed following a null model. The null model considers that the probability that any cell aij shows a presence is: (Pi / C Pj / R) / 2, where Pi is the number of presences in row i, Pj is the number of presences in column j, C is the number of columns, and R is the number of rows. We compared the 1394 degree of nestedness between carcass size categories using a standardized effect size (SES) of the NODF value. This measure indicates the number of standard deviations that the observed index is above or below the mean index of simulated matrices. We calculated the SES as a Z-score (Z observed NODF – mean simulated NODF / SD simulated NODF). Z-scores 0 indicate that the nestedness in a site was greater than the median nestedness of randomized matrices, while Z-scores 0 indicate that nestedness in a site was lower than the median nestedness of randomized matrices. We then re-calculated all these measures for each carcass size, but excluding 1) the vultures and 2) the most connected species (i.e. the species that appeared in more carcasses, whether vulture or not) from the community to evaluate if the impact of the major scavenger species on network structure varies with carcass size. In addition, we calculated quantitative matrices to compare species specialization in small, medium and large carcasses. First, to obtain a quantitative interaction matrix of the scavenging network, we calculated the minimum number of different individuals of each species that were present in each picture (n 4915 pics; Table 1). Then, we created matrices Q for each carcass type where each cell Qij represented the number of individuals of the species i that were photographed in the carcass j. Second, we calculated the degree of specialization of each species on small, medium and large carcasses. We used the d’ specialization index of Blüthgen et al. (2006) derived from the Shannon diversity index. This index ranges between 0 (no specialization) and 1 (perfect specialist). In our system, a species is highly specialized if it was found consuming a carcass that no one else consumed, and its specialization is low if it consumed carcasses that were also exploited by other species. To ascertain whether carcass size, season and section affected carcass consumption efficiency, we fitted GLMs where ‘detection time’, ‘consumption time’, ‘consumption rate’ and ‘percentage of carrion consumed’ were the response variables, and ‘carcass size’, ‘hour’, ‘season’ and ‘section’ were the categorical predictors. In this case, we used Gaussian error distributions and identity link functions. Model evaluation and selection was performed as explained above. All the GLMs were done in R 3.0.2 ( www.R-project.org/ ). Results Community richness and structure We detected a total of 18 vertebrate scavenging species (Supplementary material Appendix 1 Table A2), with the highest total species richness associated with small carcasses (Table 1, Fig. 1). Species richness curves showed that sampling effort was probably sufficient to identify most scavenger species of small and medium-sized carcasses, but that additional sampling would have detected more scavenger species at large carcasses (Supplementary material Appendix 1 Fig. A1). Scavenger richness per carcass clearly increased with carcass size (Table 1), as revealed by the GLM analyses: carcass size was included in the two best models (Table 2, 3). The most supported model also included season as predictor of scavenger richness (slightly more richness in the dry Figure 1. Spotted hyenas Crocuta crocuta (A), lions Panthera leo (B) and white-backed vultures Gypsafricanus (C) dominated both mediumsized and large carcasses, while meso-facultative scavengers such as large-spotted genets Genetta tigrina (D), white-tailed mongooses Ichneumia albicauda (E), slender mongooses Galerella sanguinea (F), and tawny eagles Aquila rapax (G) were frequent in small carcasses. All pictures were obtained by automatic cameras during the fieldwork of this study. Table 2. AICc-based model selection to assess the effect of carcass size (small, medium and large), hour of carcass placement (morning and afternoon), season (wet and dry) and section of the study area (Hluhluwe and iMfolozi) on the richness (number of species) of scavengers per carcass and the scavenging efficiency (detection time, consumption time, consumption rate and percentage of carrion biomass consumed) in Hluhluwe-iMfolozi Park, South Africa. Number of estimated parameters (k), AICc values, AICc differences (∆AICc) with the highest ranked model (i.e. the one with the lowest AICc), Akaike weights (AICcw) and the variability of the models explained by the predictors (deviance, D2) are shown. Selected models are in bold. Response variable Model k AICc ∆AICc AICcw D2 Scavenger richness size season size size season hour size season section size section size section hour season season section season hour size hour section hour season section hour section hour size season hour size season size season section season season hour size hour size season section hour season section size section hour size section hour section section hour size size hour size section size season 3 2 4 4 3 4 1 2 2 3 1 1 3 2 4 3 4 1 2 3 2 3 2 4 3 1 1 2 2 3 3 3 142.11 143.70 144.55 144.57 146.03 148.49 152.61 154.43 154.82 155.74 156.11 156.45 156.76 158.31 349.69 349.93 352.30 355.25 355.27 355.74 356.34 356.80 357.12 357.96 358.67 360.55 363.00 362.12 435.60 436.26 436.72 437.71 0.00 1.59 2.44 2.46 3.92 6.38 10.50 12.32 12.71 13.63 14.00 14.34 14.65 16.20 0.00 0.24 2.61 5.56 5.58 6.05 6.65 7.11 7.43 8.27 8.98 10.86 13.31 12.43 0.00 0.66 1.12 2.11 0.689 0.311 47.81% 37.90% 0.530 0.470 41.01% 36.73% 0.437 0.314 0.249 46.64% 48.84% 48.26% Detection time Consumption time Continued 1395 Table 2. Continued. Response variable Consumption rate % of carrion biomass consumed Model k AICc ∆AICc size section hour size season hour size season section section hour season season section section hour season hour season section hour size size season size hour size section size season hour size section hour size season section section hour season section hour season section season hour season section hour size size section hour size section size hour section hour section size season size season section hour size season hour season section hour season section season season hour 4 4 4 1 1 1 2 2 2 3 2 3 3 3 4 4 4 1 1 1 2 2 2 3 2 4 3 3 2 1 3 4 1 4 3 2 1 2 437.89 438.56 439.01 457.05 457.83 457.98 458.79 458.83 459.59 460.76 161.36 162.88 162.88 163.47 164.60 164.91 165.03 184.41 184.41 184.94 185.73 186.48 186.55 187.99 31.32 31.23 31.16 30.60 30.33 29.58 28.99 28.73 28.51 28.12 28.03 27.47 26.68 26.30 2.29 2.96 3.41 21.45 22.23 22.38 23.19 23.23 23.99 25.16 0.00 1.52 1.52 2.11 3.24 3.55 3.67 23.05 23.05 23.58 24.37 25.12 25.19 26.63 0.00 0.09 0.16 0.72 0.99 1.74 2.33 2.59 2.81 3.20 3.29 3.85 4.64 5.02 season; Table 3), although the contribution of season to the deviance of the model was low (20.72%) if compared with the contribution of carcass size (79.28%). The section of the reserve where the carcass was located did not exert any influence (Table 2). Meso-mammalian carnivores and birds of prey were the species more frequently visiting small carcasses, while large carcasses were visited typically by large facultative scavengers such as hyenas (present at 47.60%, 83.33% and 100% of small, medium and large carcasses, respectively) and lions (4.83%, 41.70% and 62.50%). Vultures and avian facultative scavengers became more frequent as carcass size increased. In contrast, mammalian meso-carnivores were absent at medium-sized and large carcasses (Fig. 2). Scavenging network structure was unaffected by carcass size, but nestedness tended to be higher at larger carcasses (Table 4). In fact, when considering medium-sized and large carcasses together (total n 21 carcasses, i.e. same n as small carcasses), the scavenging network was clearly nested. The removal of vultures and the most connected species (always the spotted hyena) produced no significant effect on the scavenging network structure if compared with the models 1396 AICcw D2 0.517 0.242 0.242 47.66% 48.74% 48.74% 0.217 0.208 0.200 0.151 0.132 0.091 15.86% 25.24% 20.33% 19.20% 13.75% 7.11% that included such species, unless medium-sized and large carcasses were joined. In this case, hyena removal, but not vulture removal, led to a significant loss of nestedness, suggesting that the hyena was mainly responsible for the nestedness of the assemblage (Table 4). Moreover, we found a negative relationship between species specialization and carcass size: d’ 0.606 0.206 (range: 0.232–0.875) for small carcasses; d’ 0.387 0.323 (range: 0.040–0.880) for medium carcasses; d’ 0.307 0.185 (range: 0.000–0.635) for large carcasses. Basically, this means that larger carcasses are shared by more species than smaller ones. Community functioning Carrion consumption efficiency was dependent on carcass size: detection time was three times longer for small than for large carcasses; consumption time was nearly four times longer for large than for small carcasses; consumption rate was 33 times higher for large than for small carcasses; percentage of carrion biomass consumed was slightly higher for small than for large carcasses (Table 5). GLM analyses supported this conclusion, as size was included in the great majority of Table 3. Selected generalized lineal models (GLMs) showing the relation between scavenger richness and scavenging efficiency (detection time, consumption time, consumption rate and percentage of carrion biomass consumed) and carcass size, hour of carcass placement season and section of the study area. The estimate of the parameters (including the sign), the standard error of the parameters (SE) and the degrees of freedom of the models (DF) are shown. Response variable Scavenger richness Model size season size Detection time size season hour size season Consumption time size size hour size section Consumption rate size size season size hour % of carrion biomass consumed size size section hour size section size hour section hour section Parameter Intercept size (medium) size (small) season (wet) Intercept size (medium) size (small) Intercept size (medium) size (small) season (wet) hour (morning) Intercept size (medium) size (small) season (wet) Intercept size (medium) size (small) Intercept size (medium) size (small) hour (morning) Intercept size (medium) size (small) section (iMfolozi) Intercept size (medium) size (small) Intercept size (medium) size (small) season (wet) Intercept size (medium) size (small) hour (morning) Intercept size (medium) size (small) Intercept size (medium) size (small) section (iMfolozi) hour (morning) Intercept size (medium) size (small) section (iMfolozi) Intercept size (medium) size (small) hour (morning) Intercept section (iMfolozi) hour (morning) Intercept section (iMfolozi) Estimate 1.778 0.595 0.891 0.379 1.558 0.517 0.889 4.475 13.109 26.992 18.503 10.018 0.566 11.653 26.556 19.358 182.990 106.610 129.800 191.94 105.12 130.80 20.89 172.82 106.82 127.26 17.97 4.454 3.283 4.317 4.802 3.428 4.363 0.487 4.250 3.317 4.294 0.477 0.791 0.040 0.151 0.869 0.046 0.136 0.081 0.074 0.831 0.041 0.141 0.069 0.817 0.045 0.148 0.060 0.968 0.098 0.085 0.926 0.086 SE DF 0.192 0.239 0.225 0.192 0.162 0.236 0.225 9.321 9.861 8.978 6.410 6.381 9.169 10.021 0.159 6.519 19.850 24.980 22.920 20.97 24.82 22.76 16.77 22.01 24.93 23.01 16.77 0.644 0.810 0.744 0.760 0.830 0.748 0.561 0.688 0.814 0.747 0.550 0.058 0.073 0.067 0.067 0.071 0.065 0.048 0.048 0.063 0.072 0.066 0.048 0.061 0.073 0.067 0.049 0.043 0.050 0.050 0.036 0.050 37 38 34 35 37 36 36 37 36 36 37 35 36 36 37 38 1397 Figure 2. Scavenger species richness per scavenger group in small, medium-sized and large carcasses. Values represent mean SD number of scavenger species per carcass (total number of scavenger species is given in parenthesis). Bubble diameter is proportional to mean values. OS: obligate scavengers (i.e. vultures); FS: facultative scavengers; LFS: large facultative scavengers; MFS: meso facultative scavengers; aFS: avian facultative scavengers; mFS: mammalian facultative scavengers. Table 4. Nestedness degree of the scavenger network per carcass size and scenario (total network, total network without vultures, and total network without the most connected species), as indicated by the NODF metrics. NODF1: nestedness of the observed matrix; NODF2: mean nestedness of the 1000 matrices simulated under the null model; Z-score: NODF standardized with the average nestedness of simulated matrices. Significant results are in bold (see main text for details). Network Small carcasses total without vultures without most connected spp. Medium-sized carcasses total without vultures without most connected spp. Large carcasses total without vultures without most connected spp. Medium-sized large carcasses total without vultures without most connected spp. 1398 NODF1 NODF2 SD p-value Z-score 20.65 22.51 13.94 17.13 4.00 (0.19) 20.56 4.49 (0.33) 13.64 3.72 (0.43) 0.879 0.434 0.081 51.93 47.06 28.30 39.90 7.68 (0.06) 45.75 9.28 (0.44) 28.04 7.39 (0.46) 1.566 0.141 0.035 63.29 51.79 53.49 51.76 7.99 (0.07) 44.87 10.25 (0.25) 46.03 8.84 (0.19) 1.443 0.675 0.844 57.63 51.77 38.98 40.31 5.26 ( 0.01) 39.86 6.55 (0.04) 32.67 5.23 (0.12) 3.290 1.818 1.206 the selected models. Moreover, the model with size only was the model with the lowest AICcw for consumption time, consumption rate and percentage of carrion biomass consumed (Table 2, 3). The hour of carcass placement exerted some influence on all the variables related to carrion consumption efficiency (Table 2): detection time, consumption time and percentage of carrion biomass consumed were lower, and consumption rate was higher, when carcasses were placed in the morning (Table 3). Detection time and consumption rate were also explained by season (Table 2): detection time was considerably longer in the wet season, while consumption rate was slightly higher in the dry season (Table 3). We also detected some influence of the park section on consumption efficiency: consumption time was slightly higher and percentage of carrion consumed lower in iMfolozi compared to Hluhluwe (Table 2, 3). Most of the carrion biomass available was consumed by facultative scavengers, especially large mammalian carnivores. Among them, hyenas were the main carrion consumers (44.59%, 48.52% and 46.83% of the biomass of small, medium-sized and large carcasses, respectively), followed by lions (0.64%, 32.15% and 23.46%). Both avian and mammalian meso-facultative scavengers consumed a considerable proportion of small carcasses. Vultures consumed near 20% of the medium-sized and large carcasses, but less than 5% of small carcasses (Fig. 3). From the pictures, the role of microbial and invertebrate decomposers in carrion biomass removal was negligible. Table 5. Consumption efficiency of each carcass type, measured as detection and consumption times (h), consumption rate (kg h1) and percentage of carrion biomass consumed. All values represent mean SD (minimum-maximum). Carcass size Small Medium Large Detection time Consumption time Consumption rate % of carrion consumed 39.11 25.80 (0.50–82.83) 20.29 17.95 (1.12–55.22) 13.47 7.52 (0.75–22.98) 53.18 28.86 (1.58–104.67) 76.38 36.50 (18.80–117.17) 182.99 108.57 (21.73–419.12) 0.14 0.31 (0.012–1.171) 1.17 1.13 (0.30–4.05) 4.45 3.91 (0.78–12.06) 94.22 10.05 (52.26–97.37) 90.67 3.14 (87.00–100) 79.14 11.68 (50.00–89.00) Discussion In accordance with our expectations, we found strong evidence that carcass size was a major factor governing the structure and functioning of scavenging assemblages. Scavenger species richness, scavenger network nestedness and all measures of scavenging efficiency (detection and consumption times, consumption rate and percentage of carrion biomass consumed) were influenced by the range in carcass size covered by our study, which varied in three orders of magnitude (from ca 2 kg to ca 4000 kg). The mean number of scavenger species exploiting a particular carcass increased with carcass size, probably because the longer temporal availability and higher conspicuousness of larger carcasses (DeVault et al. 2004) allowed more species to aggregate. However, the frequency of occurrence of small mammalian scavengers did not increase with carcass size, perhaps because large carcasses were perceived as high predation risk sites by mongooses and genets due to frequent large-predator presence. In other words, ungulate carcass sites may act as ‘hills’ or ‘hot spots’ in the ‘landscape of fear’ of these meso-carnivores (Laundré et al. 2001, Willems and Hill 2009). This is in line with other studies that found lower herbivore densities in the vicinities of dead ungulates due to higher predator occurrence (Cortés-Avizanda et al. 2009). Big predators have been recognized to limit meso-predator abundance through different intraguild interactions (Ritchie and Johnson 2009), but limiting the access to high quality food resources such as large carcasses has been overlooked. Our results regarding nestedness support the hypothesis of Sebastián-González et al. (2013): the larger the carcass, the more the interacting species and the more nested the scavenging network. Nestedness was only significant after combining medium-sized and large carcasses, although larger sample sizes would probably have led to nested patterns for each carcass size separately (Table 4). One of the probable factors favouring the nestedness–carcass size relationship is the higher number of scavengers associated Figure 3. Percentage of the total carrion biomass consumed per scavenger group in small, medium-sized and large carcasses. Bubble diameter is proportional to percentages. OS: obligate scavengers (i.e. vultures); FS: facultative scavengers; LFS: large facultative scavengers; MFS: meso facultative scavengers; aFS: avian facultative scavengers; mFS: mammalian facultative scavengers. 1399 with larger carcasses. However, this condition is not enough to explain nestedness. The null models partially accounted for the differences in matrix size by comparing the nestedness of the empiric matrix with that of randomly created matrices that preserved the number of species, carcasses and proportion of interactions. Thus, our analyses indicate that, besides the possible passive sampling effects, scavenger communities in large carcasses present a more organized structure than those in the small ones, probably due to the intervention of a sort of inter-specific interaction (Sebastián-González et al. 2013). Theoretical studies suggest that nested structures may reduce effective competition in the community (at least in mutualistic assemblages; Okuyama and Holland 2008, Bastolla et al. 2009, Sugihara and Ye 2009, Rohr et al. 2014). In such a case, the nested structure of the larger carcasses scavenging network could minimize the negative effects of competitive interactions and promote scavenger diversity to some extent. Moreover, our results indicate that nestedness in scavenging assemblages is sensitive to the most connected species in the network rather than to obligate scavengers, given that the virtual removal from the network of hyenas, but not vultures led to a loss of nestedness. This suggests that hyenas were involved in some interactions that enhance community structure, such as carcass signaling and tearing open of thick-skinned animals (Moleón et al. 2014a). It also indicates that the extinction of the spotted hyena might trigger important structural changes in the studied scavenging assemblage. The dependence of nestedness on the most connected species is also a feature of mutualistic networks (Saavedra et al. 2011). Cortés-Avizanda et al. (2012) have recently highlighted the importance of spatiotemporal unpredictability of carrion availability in promoting species diversity in scavenger guilds, but the relationship between resource unpredictability and resource size had not been addressed to date in scavenging contexts. Our analysis of quantitative matrices supported that chance may play a greater role in determining which species feeds on small carcasses than on larger carcasses. Many different species of meso-facultative scavenger exploited small carcasses in our study, probably because they had the opportunity of finding such carcasses before more narrowly selective scavengers, namely vultures, hyenas and lions, had arrived (carcass detection time decreased with carcass size). However, a low number of scavengers shared a given small carcass, as the first species to find it usually consumed most of it. In relation to carrion consumption efficiency, small carcasses were detected slower and consumed faster than larger carcasses, which could indicate an effect of carcass conspicuousness and usable biomass availability, respectively. In addition, consumption rates of larger carcasses were much higher. The fact that the percentage of carrion biomass consumed decreased as carcass size increased could be due to the higher presence of large bones that can only be partly eaten by hyenas and sometimes lions. Large facultative scavengers (especially hyenas and lions), but not vultures (i.e. the obligate scavengers), were mainly responsible for the higher carrion consumption rates. This could be the result of two interacting factors. First, maximum food intake of hyenas and lions, which are the most important scavengers among large carnivores (Pereira et al. 2014), is much higher than 1400 that of vultures: while a hyena and a lion can consume one third and one quarter of their respective body weights (i.e. ca 17–20 kg and 30–50 kg of food, respectively; according to Estes 1992), a vulture rarely eats more than 1.5 kg in a meal (Donázar 1993). Many vultures are needed, therefore, to compensate this differential per capita intake. According to population estimates in 2012, there were 303 spotted hyaenas, 200 lions and 398 breeding pairs of white-backed vulture in HiP (Howells et al. 2009–2013, D. Druce and G. Clinning pers. comm.). This would lead to maximum food intake of 6000–10 000 kg and 5151–6060 kg for the whole population of lions and hyaenas, respectively, and 1194 kg for breeding white-backed vultures. Thus, maximum consumption by the breeding sector of the vulture population meant 7.43–10.71% of the maximum food intake by lions and hyaenas, which indicates that the food demand of lions and hyenas greatly exceeded that of vultures. Second, these large carnivores can displace vultures from carcasses and successfully defend them until satiation (Moleón et al. 2014a). The influence exerted by the hour of carcass placement seems to be related with the activity rhythm of scavengers. Detection and consumption times, as well as consumption rate, diminished if carcasses were placed in the morning. This is the time of the day when vultures and other avian scavengers such as birds of prey and corvids are more active, and it is well established that avian scavengers are more efficient in finding carcasses than mammals (Wilmers et al. 2003, Ruxton and Houston 2004). In contrast, the percentage of carrion biomass that was consumed increased if carcasses were placed after midday, because hyenas, lions and other mammalian carnivores, which are able to ingest bones and other hard issues that are barely consumed by birds, forage more actively nocturnally. We also detected some spatial effect on the scavenging patterns: the section of the park (Hluhluwe and iMfolozi) contributed partly to explain differences in consumption time and percentage of carrion consumed, although its influence was considerably lower than that exerted by the size of the carcass. Finally, we found some seasonal variation in community structure and functioning: mean scavenger richness and carcass consumption rate were slightly higher during the dry season, while carcass detection time was much longer during the wet season. Several factors could explain these patterns, although their relative contribution is difficult to determine. First, less vegetation cover during the dry season probably facilitates carrion searching success of opportunistic, facultative scavengers (Ruzicka and Conover 2012). Second, rainy days could reduce the foraging activity of vultures and large raptors (Houston 1979, Selva et al. 2005) and the smell capacity of mammalian scavengers (Savage 1977) during the wet season. Comparison between predation and scavenging systems Several key size-dependent differences can be identified between the resource use patterns in scavenging versus predation networks in diverse African vertebrate systems. First, it is established that larger ungulates, especially megaherbivores, have fewer predator species than smaller ungulates (Sinclair et al. 2003, Owen-Smith and Mills 2008, Figure 4. Conceptual networks depicting hypothetical sizedependent resource use patterns in vertebrate predation and scavenging assemblages in relation to total resource pool (i.e. all prey/ carcasses into a given prey/carcass size category; (A)) and individual resources (i.e. individual prey/carcasses of a given prey/carcass size category; (B)). Black circles represent predator/scavenger species, while grey circles show the resource (dark and light grey for live prey and carrion, respectively). Grey circles size indicate resource size, i.e. ‘small’, ‘medium’ and ‘large’ prey/carcasses. A link represents the consumption of a given resource pool (A) or individual (B) by a predator/scavenger species. A) As prey size increases, less predator species are able to kill them (Sinclair et al. 2003, Owen-Smith and Mills 2008, Pereira et al. 2014). In contrast, species richness of scavengers for large carcasses is similar than that found for small carcasses (compare framed networks in the figure; present study). B) As prey size increases, more individuals die from causes besides predation (Sinclair et al. 2003, Owen-Smith and Mills 2008, Pereira et al. 2014), while all carcasses in our study area were widely exploited by scavengers (present study). Note that a given prey individual can only be eaten by one predator species; a second predator species that displaces the original predator from its kill is not predating the kill, but scavenging, or kleptoparasiting, it. In contrast, a given carcass can be scavenged by several scavenger species, in particular larger carcasses. In our study case, medium and large carcasses consumption was nearly nested (see text for further details). Pereira et al. 2014). In contrast, we have found that larger carcasses supported a richer scavenging assemblage (Fig. 4A). Second, while small and medium ungulates mostly die due to predation, megaherbivores are rarely predated and mostly when young (Sinclair et al. 2003, Owen-Smith and Mills 2008, Pereira et al. 2014). On the contrary, we found that all carcasses were subjected to scavenging, irrespective of their size, and that more links per individual resource appear in scavenging than in predation networks, especially as carcass size increases (Fig. 4B). In our system, this led to a more organized (i.e. nested) scavenging at larger carcasses (Table 4). Which are the factors behind such structural differences between predation and scavenging networks? Predator size and numbers contribute to determine live prey hunting success, so in general relatively large prey can only be killed by large social predators (Sinclair et al. 2003, Owen-Smith and Mills 2008). Larger and social scavengers have also advantages to exploit large carcasses, due to their competitive superiority and their ability to access the interior of thick-skinned animals (Moleón et al. 2014a). In contrast to very large prey, very large carcasses can be exploited by many consumer species, especially after dominant scavengers have been satiated. Small and medium-sized carcasses can be completely or mostly monopolized by a single scavenger species, but very large carcasses contain enough meat to feed more scavengers. In addition, smaller scavengers may gain access to the meat after a dominant scavenger has opened the carcass and has left (Moleón et al. 2014a). Optimal foraging theory suggests that incorporating a food resource in the diet depends on a tradeoff between the energy intake and the costs of searching and handling (capturing and manipulating) the prey (MacArthur and Pianka 1966, Pyke et al. 1977). Such costs would be much lower for carcass than for live prey consumption because capturing and manipulating efforts are absent and reduced respectively. This fact may allow for a larger number of consumers and a wider range of resource size use (Fig. 4). Besides, defending large carcasses entails further costs for predators against a wide array of potential scavengers and losses from the putrefaction and toxifying activity of decomposers (DeVault et al. 2003, Hayward et al. 2006, Moleón et al. 2014a, Pereira et al. 2014). Such costs, associated with the interactions between predators and carrion consumers (either vertebrates, invertebrates or microbes), have not been considered in previous models of optimal foraging. Concluding remarks Our findings support the idea that the particle size of the resource affects the structure and functioning of vertebrate scavenging networks. The relevance of the size of the food resource had been hardly recognized until now in a scavenging context, despite being pervasive in other trophic assemblages (Woodward et al. 2005). Wide size variation of available carcasses allows a wide spectrum of species to differentially access the carrion resource, which might promote scavenger diversity. We have explicitly shown that the scavenger assemblage is more organized as carcass size increases, but full understanding of the mechanisms and consequences of nestedness in scavenging and other competitive networks requires detailed investigation. Also, we have highlighted the pivotal role that large facultative scavengers play in the structure and functioning of diverse scavenging networks. Finally, we have identified several size-dependent differences in scavenging networks structure compared with predator–prey systems. For predators, the costs associated with competition from carrion consumers might be an important feature limiting the benefits of capturing large live prey. The consequences of carcass size variation over time and space for scavenging networks deserve further theoretical and applied research effort, especially in the light of the current scenario of global environmental change (DeVault et al. 2003, Wilson and Wolkovich 2011, Moleón et al. 2014a, Pereira et al. 2014). Our experimental provision of carrion needs to be complemented by future studies on the carrion naturally available, quantifying live prey versus carrion intake by facultative scavengers and recognizing that animal deaths are frequently hastened through the agency of a predator. 1401 Acknowledgements – G. Clinning and D. Druce decisively helped with fieldwork logistics. J. Bautista, J. R. Fernández-Navarro, D. Carmona-López, E. Ramos, M. S. García-Heras, E. McCain, Thamalina, L. Munro, C. Geoghegan, H. Druce, E. Virgós, S. Cabezas-Díaz, J. Lozano, N. Yelo, S. Eguía, I. Moleón, L. Moleón, E. Moleón, E. Moleón, S. Justicia, A. J. Paiz, E. Moyano, J. M. Molina, C. Hue, B. Whittington-Jones, J. Bird, as well as several rangers and personnel at Hluhluwe Research Centre and the abattoirs kindly helped at different fieldwork stages. We also thank Ezemvelo KZN Wildlife for permissions and logistic facilities. The study was partly funded by the Spanish Ministry of Economy and Competitiveness through grant CGL2012-40013-C02-02. MM was supported by a postdoctoral fellowship from the Spanish Ministry of Education (Plan Nacional de I D i 2008-2011). ES-G benefitted from a São Paulo Foundation grant from Brazil (Ref.: 2011/17968-2). References Almeida-Neto, M. et al. 2008. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. – Oikos 117: 1227–1239. Barton, P. S. et al. 2013. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. – Oecologia 171: 761–772. Bastolla, U. et al. 2009. The architecture of mutualistic networks minimizes competition and increases biodiversity. – Nature 458: 1018–1020. Beasley, J. C. et al. 2012. Carrion cycling in food webs: comparisons among terrestrial and marine ecosystems. – Oikos 121: 1021–1026. Blázquez, M. et al. 2009. Spatio-temporal segregation of facultative avian scavengers at ungulate carcasses. – Acta Oecol. 35: 645–650. Blüthgen, N. et al. 2006. Measuring specialization in species interaction networks. – BMC Ecol. 6: 9. Brooks, P. M. and Macdonald, I. A. W. 1983. The Hluhluwe– Umfolozi Reserve: an ecological case history. – In: Owen-Smith, R. N. (ed.), Management of large mammals in African conservation areas. Haum Educational Publishers, pp. 51–77. Burnham, K. P. and Anderson, D. R. 2002. Model selection and multimodel inference. A practical information-theoretic approach. – Springer. Burnham, K. P. and Anderson, D. R. 2004. Multimodel inference: understanding AIC and BIC in model selection. – Sociol. Meth. Res. 33: 261–304. Cohen, J. E. et al. 1993. Body sizes of animal predators and animal prey in food webs. – J. Anim. Ecol. 62: 67–78. Cortés-Avizanda, A. et al. 2009. Effects of carrion resources on herbivore spatial distribution are mediated by facultative scavengers. – Basic Appl. Ecol. 10: 265–272. Cortés-Avizanda, A. et al. 2012. Resource unpredictability promotes species diversity and coexistence in an avian scavenger guild: a field experiment. – Ecology 93: 2570–2579. DeVault, T. L. and Rhodes Jr., O. E. 2002. Identification of vertebrate scavengers of small mammal carcasses in a forested landscape. – Acta Theriol. 47: 185–192. DeVault, T. L. et al. 2003. Scavenging by vertebrates: behavioural, ecological and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. – Oikos 102: 225–234. DeVault, T. L. et al. 2004. Factors influencing the acquisition of rodent carrion by vertebrate scavengers and decomposers. – Can. J. Zool. 82: 502–509. Donázar, J. A. 1993. Los buitres ibéricos. Biología y conservación. – J. M. Reyero Editor. 1402 Estes, R. D. 1992. The behavior guide to African mammals: including hoofed mammals, carnivores, primates. – Univ. of California Press. Grange, S. et al. 2012. Changes of population trends and mortality patterns in response to the reintroduction of large predators: the case study of African ungulates. – Acta Oecol. 42: 16–29. Guimarães, P. R. and Guimarães, P. 2006. Improving the analyses of nestedness for large sets of matrices. – Environ. Modell. Softw. 21: 1512–1513. Hayward, M. W. et al. 2006. Prey preferences of the cheetah (Acinonyx jubatus) (Felidae: Carnivora): morphological limitations or the need to capture rapidly consumable prey before kleptoparasites arrive? – J. Zool. (Lond.) 270: 615–627. Houston, D. C. 1979. The adaptations of scavengers. – In: Sinclair, A. R. E. and Griffiths, M. N. (eds), Serengeti, dynamics of an ecosystem. Univ. of Chicago Press, pp. 263–286. Howells, B. et al. 2009–2013. Aerial survey of Zululand tree nesting vultures. Internal EKZNW Reports. – Ezemvelo KZN Wildlife. James, F. C. and McCulloch, C. E. 1990. Multivariate analysis in ecology and systematics: panacea or pandora’s box. – Annu. Rev. Ecol. Syst. 21: 129–166. Kendall, C. et al. 2012. Mechanisms of coexistence in vultures: understanding the patterns of vulture abundance at carcasses in Masai Mara National Reserve, Kenya. – Condor 114: 523–531. Laundré, J. W. et al. 2001. Wolves, elk and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. – Can. J. Zool. 79: 1401–1409. MacArthur, R. H. and Pianka, E. R. 1966. On optimal use of a patchy environment. – Am. Nat. 100: 603–609. Magurran, A. E. 1991. Ecological diversity and its measurement. – Chapman and Hall. Moleón, M. et al. 2014a. Inter-specific interactions linking predation and scavenging in terrestrial vertebrate assemblages. – Biol. Rev. 89: 1042–1054. Moleón, M. et al. 2014b. Humans and scavengers: the evolution of interactions and ecosystem services. – BioScience 64: 394–403. Okuyama, T. and Holland, J. N. 2008. Network structural properties mediate the stability of mutualistic communities. – Ecol. Lett. 11: 208–216. Olson, Z. H. et al. 2012. Scavenger community response to the removal of a dominant scavenger. – Oikos 121: 77–84. Owen-Smith, N. and Mills, M. G. L. 2008. Predator–prey size relationships in an African large-mammal food web. – J. Anim. Ecol. 77: 173–183. Pereira, L. M. et al. 2014. Facultative predation and scavenging by mammalian carnivores: seasonal, regional and intra-guild comparisons. – Mammal Rev. 44: 44–45. Pyke, G. H. et al. 1977. Optimal foraging: a selective review of theory and tests. – Q. Rev. Biol. 52: 137–154. Ritchie, E. G. and Johnson, C. N. 2009. Predator interactions, mesopredator release and biodiversity conservation. – Ecol. Lett. 12: 982–998. Rohr, R. P. et al. 2014. On the structural stability of mutualistic systems. – Science 345: 1253497. Ruxton, G. D. and Houston, D. C. 2004. Obligate vertebrate scavengers must by large soaring fliers. – J. Theor. Biol. 228: 431–436. Ruzicka, R. E. and Conover, M. R. 2012. Does weather or site characteristics influence the ability of scavengers to locate food? – Ethology 118: 187–196. Saavedra, S. et al. 2011 Strong contributors to network persistence are the most vulnerable to extinction. – Nature 478: 233–235. Savage, R. J. G. 1977. Evolution in carnivorous mammals. – Palaeontology 20: 237–271. Sebastián-González, E. et al. 2013. Interactive effects of obligate scavengers and scavenger community richness on lagomorph carcass consumption patterns. – Ibis 155: 881–885. Selva, N. 2004. The role of scavenging in the predator community of Białowieża Primeval Forest (E Poland). – PhD thesis, Univ. de Sevilla. Selva, N. and Fortuna, M. A. 2007. The nested structure of a scavenger community. – Proc. R. Soc. B 274: 1101–1108. Selva, N. et al. 2003. Scavenging on European bison carcasses in Bialowieza Primeval Forest (eastern Poland). – Ecoscience 10: 303–311. Selva, N. et al. 2005. Factors affecting carcass use by a guild of scavengers in European temperate woodland. – Can. J. Zool. 83: 1590–1601. Sinclair, A. R. E. et al. 2003. Patterns of predation in a diverse predator–prey community. – Nature 425: 288–290. Sugihara, G. and Ye, H. 2009. Cooperative network dynamics. – Nature 458: 979–980. Ulrich, W. et al. 2009. A consumer’s guide to nestedness analysis. – Oikos 118: 3–17. Willems, E. P. and Hill, R. A. 2009. Predator-specific landscapes of fear and resource distribution: effects on spatial range use. – Ecology 90: 546–555. Wilmers, C. C. et al. 2003. Resource dispersion and consumer dominance: scavenging at wolf and hunter-killed carcasses in Greater Yellowstone. – Ecol. Lett. 6: 996–1003. Wilson, E. E. and Wolkovich, E. M. 2011. Scavenging: how carnivores and carrion structure communities. – Trends Ecol. Evol. 26: 129–135. Woodward, G. et al. 2005. Body size in ecological networks. – Trends Ecol. Evol. 20: 402–409. Supplementary material (available online as Appendix oik.02222 at www.oikosjournal.org/readers/appendix ). Appendix 1. Table A1. Weights of the ungulate species considered in this study. Table A2. Scavenger species detected in this study, according to carcass size. Figure A1. Species richness curves. 1403