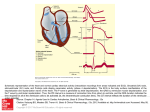
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Intrinsic changes on automatism, conduction
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Coronary artery disease wikipedia , lookup
Jatene procedure wikipedia , lookup
Electrocardiography wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
J Appl Physiol 92: 225–229, 2002. Intrinsic changes on automatism, conduction, and refractoriness by exercise in isolated rabbit heart L. SUCH,1 A. RODRIGUEZ,1 A. ALBEROLA,1 L. LOPEZ,1 R. RUIZ,1 L. ARTAL,1 I. PONS,1 M. L. PONS,1 C. GARCÍA,2 AND F. J. CHORRO3 Departments of 1Physiology, 2Physiotherapy, and 3Medicine, University of Valencia, 46010 Valencia, Spain Received 3 February 2000; accepted in final form 29 August 2001 HR (17). The concept of an increase in the parasympathetic activity by training is supported by the observations of increased acetylcholine levels in the myocardium of trained animals (2), as well as a greater gain of baroreceptive mechanisms in trained animals (1). Conversely, several reports support the fact that electrophysiological training-induced modifications are the result of intrinsic mechanisms. Indeed, Katona et al. (10) and Lewis et al. (11) concluded that traininginduced bradycardia at rest is caused by a reduction in the intrinsic cardiac rate and not the result of an increase in parasympathetic tone. Finally, it has been reported that, in some cases, the electrophysiological changes produced by training are related to some heart modifications, such as ventricular hypertrophy (3) or myocardial damage (12, 15). The main objective of the present study was to investigate in isolated rabbit heart (Langendorff preparation), and thus not submitted to nervous and/or humoral influences, the effect of exercise training on sinus chronotropism, sinus nodeatrial conduction, atrioventricular (A-V) node conduction and refractoriness, ventriculoatrial (V-A) retrograde conduction and refractoriness, and ventricular refractoriness to analyze the intrinsic modifications of these parameters. MATERIALS AND METHODS REGULARLY PERFORMED ENDURANCE exercise results in changes in several cardiovascular variables at rest, and especially significant are the changes on automatism and conduction. Several studies have assessed the contribution of both the autonomic nervous system and the intrinsic mechanisms on the myocardial changes observed in trained humans and laboratory animals (1, 10). Some authors support the fact that the reduction in heart rate (HR) at rest as a consequence of endurance training is the result of an increased vagal tone (18, 19) and not because of a reduction in the intrinsic Two groups of New Zealand rabbits were studied. Ten rabbits (trained group) were trained on motor-driven treadmills, and eight rabbits (control group) were housed in the animal quarters for 46 days. In the trained group, after familiarization with treadmills during 4 days, rabbits were submitted to a running program. They ran for 5 days/wk for 6 wk at 0.5 m/s. Each one of the training sessions was divided into six periods of 4 min of running and 1 min of rest. The correct realization of treadmill exercise was constantly supervised, and those animals that did not adequately run on the treadmill, because they either stopped frequently or ran irregularly, were excluded from the study. The number of animals excluded for these reasons was two, and eight animals responded well to the treadmill protocol. Preparation and perfusion. Sixteen isolated rabbit hearts were studied. All of the procedures were performed in accor- Address for reprint requests and other correspondence: L. Such, Departamento de Fisiologı́a, Facultad de Medicina y Odontologı́a, Av. Blasco Ibáñez, 17, 46010 Valencia, Spain (E-mail: Luis.Such @uv.es). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. physical training; heart electrophysiology http://www.jap.org 8750-7587/02 $5.00 Copyright © 2002 the American Physiological Society 225 Downloaded from http://jap.physiology.org/ by 10.220.33.4 on April 5, 2017 Such, L., A. Rodriguez, A. Alberola, L. Lopez, R. Ruiz, L. Artal, I. Pons, M. L. Pons, C. Garcı́a, and F. J. Chorro. Intrinsic changes on automatism, conduction, and refractoriness by exercise in isolated rabbit heart. J Appl Physiol 92: 225–229, 2002.—We have studied the intrinsic modifications on myocardial automatism, conduction, and refractoriness produced by chronic exercise. Experiments were performed on isolated rabbit hearts. Trained animals were submitted to exercise on a treadmill. The parameters investigated were 1) R-R interval, noncorrected and corrected sinus node recovery time (SNRT) as automatism index; 2) sinoatrial conduction time; 3) Wenckebach cycle length (WCL) and retrograde WCL, as atrioventricular (A-V) and ventriculoatrial conduction index; and 4) effective and functional refractory periods of left ventricle, A-V node, and ventriculoatrial retrograde conduction system. Measurements were also performed on coronary flow, weight of the hearts, and thiobarbituric acid reagent substances and glutathione in myocardium, quadriceps femoris muscle, liver, and kidney, to analyze whether these substances related to oxidative stress were modified by training. The following parameters were larger (P ⬍ 0.05) in trained vs. untrained animals: R-R interval (365 ⫾ 49 vs. 286 ⫾ 60 ms), WCL (177 ⫾ 20 vs. 146 ⫾ 32 ms), and functional refractory period of the left ventricle (172 ⫾ 27 vs. 141 ⫾ 5 ms). Corrected SNRT was not different between groups despite the larger noncorrected SNRT obtained in trained animals. Thus training depresses sinus chronotropism, A-V nodal conduction, and increases ventricular refractoriness by intrinsic mechanisms, which do not involve changes in myocardial mass and/or coronary flow. 226 TRAINING AND CARDIAC INTRINSIC ELECTROPHYSIOLOGY J Appl Physiol • VOL of 30-s duration were used to calculate the SNRT (the first train at a frequency 10% above the spontaneous HR), and in each stimulation period the train frequency was increased by 40 beats/min. Several trains were used until the maximum frequency with auricular capture was reached. The pause between two consecutive trains was 30 s. 2) Atrial stimulation with a short train of eight stimuli at a rate slightly higher than spontaneous HR (Narula test) was used to determine SACT. 3) Pacing with fixed-rate trains of 30-s duration (stimuli of 2-ms duration and twice diastolic threshold) at increasing rates (10-beat/min increments) was used to calculate the WCL. 4) Atrial extrastimulus test was used with basic trains of 10 beats at a cycle length of 250 ms, which was a frequency slightly higher than the spontaneous frequency in the control group, and the pause between trains was 1 s. This basic cycle length was maintained in the trained group, and the extrastimulus was delivered with 5-ms decrements of the coupling interval, from a 250-ms coupling interval to determine the AVNERP, and A-V node functional refractory period. The left ventricle was stimulated in a random sequence, and the protocol was continued. 5) Pacing with fixed-rate trains of 30-s duration (stimuli of 2-ms duration and twice diastolic threshold) at increasing rates (10-beat/min increments) was used to calculate RWCL. 6) Ventricular extrastimulus test, using the same technique as in atrial extrastimulus test, was used to determine ventricular and V-A retrograde conduction refractoriness. Spontaneous HR was measured in all cases, immediately before the electrophysiological study. Measurements were also performed on thiobarbituric acid reagent substances (TBARS) and GSH, in myocardium, quadriceps femoris muscle, liver, and kidney, to analyze whether these substances that are related to the oxidative stress were modified by our training method. Immediately after the animals were killed, biopsies (between 800 and 1,000 mg) were taken from the quadriceps femoris muscle, liver, and kidney. At the end of the electrophysiological measurements, an additional biopsy of the myocardium was taken to analyze the substances mentioned above. Tissue samples were immediately placed in liquid nitrogen and manually powdered with a mortar. Part of this powder was homogenized in cold 6% perchloric acid containing 1 mM EDTA, and GSH was determined with 1-dichloro-2,4-dinitrobenzene as substrate and glutathione S-transferase, according to Brigelius et al. (5). Another part of the powder was homogenized with cold 1.15% KCl and afterwards was mixed with an aqueous solution of phosphoric and thiobarbituric acids and boiled. After extraction with butanol, measurements were made by a spectrophotometric method according to Uchiyama and Mishara (23). Coronary flow was measured directly by collection during 1 min. Measurements were performed before to start the electrophysiological protocol and also at the end of this period. The hearts were weighed at the end of the experiments. Table 1. Parameters of chronotropism and conduction R-R SNRT CSNRT SACT WCL RWCL Control 286⫾60 460⫾36 174⫾68 25⫾15 146⫾20 179⫾25 n 8 8 8 8 7 8 Trained 365⫾49* 554⫾66* 189⫾45 31⫾22 177⫾32* 205⫾32 n 8 8 8 7 8 7 Values are means ⫾ SD in ms; n ⫽ no. of experiments. R-R, R-wave to R-wave; SNRT, sinus node recovery time; CSNRT, corrected SNRT; SACT, sinoatrial conduction time; WCL, Wenckebach cycle length; RWCL, retrograde WCL. * P ⬍ 0.05 vs. control. 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.4 on April 5, 2017 dance with the agreements of the European Convention of Strasbourg of March 18, 1986 (Instrument of Ratification of 8/2/89, Official State Bulletin of 10/25/90). After heparinization, the animals were killed by cervical dislocation, and, after midsternal thoracotomy, the heart was quickly removed and immersed in cold Tyrode solution for further preparation. The aorta was cannulated and connected to a Langendorff system to provide the heart with warmed and oxygenated Tyrode solution containing (in mM) 130 NaCl, 4.7 KCl, 2.2 CaCl2, 0.6 MgCl2, 1.2 NaH2PO4, 24.2 NaHCO3, and 12 glucose. The pH was maintained at 7.4 by equilibration with a mixture of 95% O2 and 5% CO2. The temperature was constant throughout the experiment (37°C), and perfusion pressure was maintained at 50 mmHg. Four bipolar surface electrodes (silver wire Teflon coated, with an interelectrode distance of 1 mm) were positioned as follows: two on the right atrium and two on the left ventricle for pacing and recording. The right atrium was opened to place one bipolar electrode on the septal leaflet of the tricuspid valve for His bundle electrogram recording, as well as low right atrial activity and high-ventricular septal depolarization. After amplification, the electrograms were recorded by a four-channel Mingograph 34 polygraph (Siemens-Elema, Solna, Sweden). Electrical stimuli were delivered by a Grass S-88 stimulator (Grass Instruments, Quincy, MA) fitted to two SIU 5 stimulus isolation units. The recordings were made at 100 mm/s, with a measurement precision of 1 ms. In previous studies, our laboratory checked the reproducibility of the measurements (16, 17). Measurements and calculations. The procedure in the experiments with trained (trained group) and nontrained rabbits (control group) was the same. The parameters studied and their definitions were as follows: 1) R-R interval: the interval between two successive ventricle electrograms during basal sinus rhythm; 2) sinus node recovery time (SNRT): the A1-A2 cycle length after atrial pacing (A1 represents the last beat of an atrial pacing train, and A2 the first spontaneous beat after pacing); 3) corrected SNRT (CSNRT): SNRT ⫺ basal sinus cycle length; 4) sinoatrial conduction time (SACT): 0.5 ⫻ (return cycle length ⫺ basal sinus cycle length); 5) Wenckebach cycle length (WCL): the maximum cycle length of atrial pacing that produces A-V Wenckebach block; 6) retrograde WCL (RWCL): the maximum cycle length of ventricular pacing that impedes a 1:1 V-A conduction; 7) ventricular effective refractory period: the maximum ventricular extrastimulus coupling interval without ventricular capture (S1-S2 interval; S1 and S2 represent the stimulus artifacts of the last beat of the basic train and the extrastimulus, respectively); 8) ventricular functional refractory period: the minimum interval between the ventricle electrogram produced by the last basic ventricular train stimulus and that triggered by the extrastimulus; 9) A-V node effective refractory period (AVNERP): the maximum atrial electrogram coupling interval, produced by the extrastimulus, without His bundle capture; 10) A-V node functional refractory period (AVNFRP): the minimum interval between the His electrogram produced by the last basic atrial train stimulus and that triggered by the extrastimulus; 11) effective refractory period of the V-A retrograde conduction: the maximum ventricular electrogram coupling interval, produced by the extrastimulus, without atrial capture; 12) functional refractory period of the V-A retrograde conduction: the minimum interval between the atrial electrogram produced by the last basic ventricular train stimulus and that triggered by the ventricular extrastimulus. The right atrium was stimulated in a random sequence according to the following protocol. 1) Fixed-frequency trains 227 TRAINING AND CARDIAC INTRINSIC ELECTROPHYSIOLOGY Table 2. A-V nodal, V-A retrograde conduction, and ventricular refractoriness Control n Trained n AVNERP AVNFRP VARCERP VARCFRP VERP VFRP 110 ⫾ 19 5 116 ⫾ 26 5 152 ⫾ 18 8 157 ⫾ 23 8 146 ⫾ 30 5 175 ⫾ 16 5 177 ⫾ 14 7 186 ⫾ 26 6 129 ⫾ 30 5 153 ⫾ 27 5 141 ⫾ 5 5 172 ⫾ 27* 5 Values are means ⫾ SD in ms; n ⫽ no. of experiments. AVNERP, atrioventricular (A-V) node effective refractory period; AVNFRP, A-V node functional refractory period; VARCERP, ventriculoatrial (V-A) retrograde conduction effective refractory period; VARCFRP, V-A retrograde conduction functional refractory period; VERP, ventricular effective refractory period; VFRP, ventricular functional refractory period. * P ⬍ 0.05 vs. control. Heart weights were similar in trained and control rabbits (12.1 ⫾ 1.9 and 11.8 ⫾ 1.9 g, respectively). RESULTS DISCUSSION Sinus chronotropism and sinoatrial conduction. The R-R interval was 28% larger in the trained vs. control group, and SNRT was 20% larger in the trained group vs. control group (Table 1). No differences were observed in CSNRT and SACT between the two groups of animals. A-V and V-A conduction. WCL was 21% larger in the trained group with respect to the control group (Table 1). Ventricular, A-V nodal, and V-A retrograde conduction system refractoriness. The functional refractory period of the left ventricle was 20% larger in trained animals vs. controls. No significant differences were observed in the remaining parameters of refractoriness between trained and untrained animals (Table 2). AVNERP was not measured in three cases because, in these animals, the atrial effective refractory period was reached before the A-V node refractory period took place. Ventricular refractoriness was only measured in five experiments because the extrastimulus artifacts or the electrograms were not adequately identified in three cases. GSH and TBARS content. No significant differences in GSH and TBARS content were observed between groups (Table 3). Coronary flow and heart weights. Coronary flow was not different between groups when it was normalized with respect to HR and thus expressed in milliliters per minute per gram per heartbeat (0.021 ⫾ 0.06 vs. 0.021 ⫾ 0.005 ml 䡠 min⫺1 䡠 g⫺1 䡠 heartbeat⫺1), despite the differences when it was expressed in milliliters per minute per gram (3.1 ⫾ 0.9 in trained group vs. 4.4 ⫾ 1.3 ml 䡠 min⫺1 䡠 g⫺1 in control animals). We have investigated the effects of physical training on the electrophysiological properties of the heart by using an isolated rabbit heart preparation. With respect to the experimental preparation used, two main points should be considered. First, it has been reported that the rabbit model is ideal for examining the effects of exercise training, and, by using the proper intensity, duration, and frequency of exercise, the rabbit obtains a documented cardiovascular training effect rather easily (8). Second, the isolated heart preparation used is not a “working heart,” and the differences between groups are not due to differences in cardiac work. Our results demonstrate that physical training produces electrophysiological modifications in the isolated rabbit heart. These changes take place not only on the chronotropism but also on conduction. Modifications on refractoriness were not statistically significant, except for the changes observed in ventricular functional refractory period. It has been previously reported that training increases parasympathetic activity (1, 7, 18, 19). Other studies show that electrophysiological training-induced modifications are the result of intrinsic mechanisms (10, 11). In our study, the chronotropic-negative effect of training is shown by the decrease in HR. CSNRT did not change, despite the increase in noncorrected SNRT, and this, therefore, does not imply prolongation in this parameter. The increase in noncorrected SNRT is probably due to a decrease in HR. The changes observed in the intrinsic HR are in accordance with previous experimental studies performed in rats (13) and dogs (14). However, other studies in isolated Table 3. GSH and TBARS in different tissues Heart Control n Trained n Muscle Liver Kidney TBARS GSH TBARS GSH TBARS GSH TBARS GSH 54 ⫾ 7 7 61 ⫾ 24 6 1.2 ⫾ 0.2 7 0.9 ⫾ 0.2 6 18 ⫾ 6 7 19 ⫾ 12 6 0.9 ⫾ 0.2 6 0.9 ⫾ 0.2 6 32 ⫾ 17 7 37 ⫾ 14 6 8.4 ⫾ 1.1 7 7.3 ⫾ 0.8 6 53 ⫾ 19 7 75 ⫾ 39 6 1.2 ⫾ 0.3 7 0.9 ⫾ 0.4 6 Values are means ⫾ SD; n ⫽ no. of experiments. GSH values are in mol/g. Thiobarbituric acid reagent substance (TBARS) values are expressed as optical density at 535 nm (OD535) minus optical density at 520 nm (OD520). J Appl Physiol • VOL 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.4 on April 5, 2017 Data analysis. Student’s unpaired t-test was used to perform comparisons between the two groups. Statistical significance was accepted when P ⬍ 0.05. 228 TRAINING AND CARDIAC INTRINSIC ELECTROPHYSIOLOGY J Appl Physiol • VOL and exerts deleterious effects on tissues (4, 6, 9). Thus measurements were performed in this study to assess whether the training program used produced modifications in lipid peroxidation (TBARS) and in the GSH content in several tissues. We did not find any modification of TBARS and GSH content by training, which is in agreement with other studies showing that neither long-term physical training nor acute exhaustive exercise affected lipid peroxidation (24). Similarly, others did not find modifications by training of the antioxidant enzymatic activity in several tissues (21). Thus lipid peroxidation does not appear to be involved in the electrophysiological modifications mentioned above. Finally, it seems that the electrophysiological modifications by training are not related to coronary flow changes, because this was not modified in trained animals, despite the decrease in nonnormalized coronary flow, which is an indirect effect closely related to the decrease in HR. In conclusion, we demonstrate in the present study that training endurance in rabbits produces a depression on sinoatrial node automatism and A-V node conduction and some modifications on ventricular refractoriness by means of intrinsic mechanisms. These mechanisms do not seem to be related to heart hypertrophy, lipid peroxidation, and/or coronary flow modifications. The authors express sincere thanks to Dr. Lluch for valuable review and advice on the manuscript. REFERENCES 1. Bedford TG and Tipton CM. Exercise training and the arterial baroreflex. J Appl Physiol 63: 1926–1932, 1987. 2. Bjornstad H, Storstein L, Dyre Meen H, and Hals O. Electrocardiographic findings of heart rate and conduction times in athletic students and sedentary control subjects. Cardiology 83: 258–267, 1993. 3. Bjornstad H, Storstein L, Dyre Meen H, and Hals O. Electrocardiographic finding of left, right and septal hypertrophy in athletic students and sedentary controls. Cardiology 82: 56–65, 1993. 4. Brady PSL, Brady J, and Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. J Nutr 109: 1103–1109, 1979. 5. Brigelius R, Muckel C, Akerboom TPM, and Sies H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol 32: 2529–2534, 1983. 6. Davies CTM and White MJ. Muscle weakness following eccentric work in men. Pflügers Arch 392: 168–171, 1981. 7. De Schryver C and Mertens-Strythagen J. Heart tissue acetylcholine in chronically exercised rats. Experientia 31: 316– 318, 1975. 8. DiCarlo SE and Bishop VS. Exercise training enhances cardiac afferent inhibition of baroreflex function. Am J Physiol Heart Circ Physiol 258: H212–H220, 1990. 9. Dillard CJ, Litov RE, Savin WM, Dumelin EE, and Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol 45: 927–932, 1978. 10. Katona PG, McLean M, Dighton DH, and Guz A. Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. J Appl Physiol 52: 1652–1657, 1982. 11. Lewis SF, Nylander E, Gad P, and Areskog N. Non-autonomic component in bradycardia of endurance trained men at rest and during exercise. Acta Physiol Scand 109: 297–305, 1980. 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.4 on April 5, 2017 hearts from trained animals did not find significant changes in HR (22). The intrinsic component of the bradycardia of trained subjects has been related to cardiac enlargement and to the tonic stretch of the sinoatrial pacemaker, which could produce the atrial dilatation of prolonged endurance training (11). A modification in cardiac myocyte metabolism that resulted in more efficient energy utilization and generation has also been suggested as a cause of intrinsic bradycardia (10). It has also been reported that, in some cases, bradycardia is sick sinus syndrome dependent (12). We did not find any relationship between the intrinsic bradycardia and myocardial hypertrophy, because there was no difference in heart weight between trained and control rabbits; thus other mechanisms may be taking place in our results. With respect to the effect of training on A-V nodal conduction, our study shows that chronic exercise also produces a depression on A-V nodal conduction because the WCL increased in trained animals. RWCL tended to increase, although the differences did not reach statistical significance (P ⫽ 0.09), thus indicating a possible effect of training on retrograde V-A nodal conduction. These results are in agreement with previous reports that have shown a depression on A-V conduction in athletes that evidenced first degree and, to a lesser extent, second degree of A-V block (20, 25, 26). The depressing effects of physical training on A-V nodal conduction have been attributed to an increased vagal tone (2, 12). Our results indicate the intervention of an intrinsic component in the alteration of A-V conduction. Several proposals have been given to explain the A-V conduction intrinsic changes due to training. Bjornstad et al. (3) reported a relationship between PQ prolongation and left ventricular hypertrophy, which was in good accordance with the increased incidence of A-V block I in subjects with left ventricular hypertrophy (3). Other authors support the view that A-V block in athletes is caused by myocardial damage (reviewed in Ref. 3). Finally, our results did not show any relationship between the increase in the WCL and myocardial hypertrophy, and other mechanisms may be involved. With respect to refractoriness, the only effect found in the present work was an increase in the ventricular functional refractory period. The possible contribution of A-V node refractoriness to the increase of conduction block cycle length by training has not been demonstrated in the present study, because, as shown in RESULTS, the A-V node refractory periods were not significantly modified by training. Nevertheless, training could also facilitate the appearance of the nodal fatigue phenomenon, which is known to be involved in the production of A-V block by atrial stimulation at increasing rates. The small number of refractory periods measured could also contribute to the explanation that the differences in refractoriness between trained and control animals did not reach statistical significance, especially in the ventricular effective refractory period. Several studies have shown that exercise can induce free-radical production, promotes lipid peroxidation, TRAINING AND CARDIAC INTRINSIC ELECTROPHYSIOLOGY 12. Northcote RJ, Gordon PC, and Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br Heart J 61: 155–160, 1989. 13. Nylander E, Sigvardsson K, and Kilbom A. Training-induced bradycardia and intrinsic heart rate in rats. Eur J Appl Physiol 48: 189–199, 1982. 14. Ordway GA, Charles JB, Randall DC, Billman GE, and Wekstein DR. Heart rate adaptation to exercise training in cardiac-denervated dogs. J Appl Physiol 52: 1586–1590, 1982. 15. Palatini P, Marablino G, and Sperti G. Prevalence and possible mechanisms of ventricular arrythmias in athletes. Am Heart J 110: 560–567, 1985. 17. Sanchis J, Chorro FJ, Such L, Matamoros J, Cortina J, and López Merino V. Effect of site, summation and asynchronism of inputs on atrioventricular nodal conduction and refractoriness. Eur Heart J 14: 1421–1426, 1993. 16. Sanchis J, Chorro FJ, Such L, Artal L, Bodı́ V, Atienza F, Llavador E, Llavador J, and López Merino V. Recovery curve and concealed conduction in the His-Purkinje system of the rabbit heart: effects of radiofrequency modification of the low AV junction. Pacing Clin Electrophysiol 19: 31–41, 1996. 18. Seals DR and Chase PB. Influence of physical training on HR variability and baroreflex circulatory control. J Appl Physiol 66: 1886–1895, 1989. 229 19. Shi X, Stevens GHJ, Foresman BH, Stern SA, and Raven PB. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc 27: 1406–1413, 1995. 20. Talan DA, Bauernfeind RA, Ashley WW, Kanakis C, and Rosen KM. Twenty-four hour continuous ECG recordings in long distance runners. Chest 1: 19–24, 1982. 21. Tiidus PM and Houston ME. Antioxidant and oxidative enzyme adaptations to vitamin E deprivation and training. Med Sci Sports Exerc 26: 354–359, 1994. 22. Tipton CM, Matthes RD, Tcheng T, Dowell RT, and Vailas AC. The use of Langendorff preparation to study the bradycardia of training. Med Sci Sports Exerc 9: 220–230, 1977. 23. Uchiyama M and Mishara M. Determination of malondyaldehide precursor in tissues by thiobarbiturate acid test. Anal Biochem 86: 271–278, 1977. 24. Viinikka L, Vuori J, and Ylikorkala O. Lipid peroxides, prostacyclin, and thromboxane A2 in runners during acute exercise. Med Sci Sports Exerc 16: 275–277, 1984. 25. Viitasalo MT, Kala R, and Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J 47: 213–220, 1982. 26. Viitasalo MT, Kala R, and Eisalo A. Ambulatory electrocardiographic findings in young athletes between 14–16 years of age. Eur Heart J 5: 2–6, 1984. Downloaded from http://jap.physiology.org/ by 10.220.33.4 on April 5, 2017 J Appl Physiol • VOL 92 • JANUARY 2002 • www.jap.org