* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Doc - Medtronic
Survey
Document related concepts
Transcript
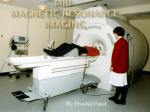
BACKGROUNDER Advisa DR MRI™ SureScan®Pacing System The Medtronic Advisa DR MRI™ SureScan® pacing system is Medtronic’s secondgeneration MR-Conditional pacemaker and is the first to combine the most advanced pacing technology with proven MRI access. MR-Conditional is a term used to indicate that a medical device may be used in the MRI under certain conditions, such as a particular type of MRI scanner and scanner settings. Features with Advisa MRI Advisa MRI comes with several innovative enhancements to provide patients and physicians with the most advanced pacing technology while mitigating hazards produced by the MRI environment. Since MRI scanners may cause traditional pacemakers to misinterpret MRI-generated electrical noise and withhold pacing therapy or deliver unnecessary pacing therapy, the SureScan features sets the device into an appropriate mode for the MRI environment. Other features include: MVP® (Managed Ventricular Pacing) algorithm, which is proven to reduce unnecessary ventricular pacing.i Complete automaticity with Ventricular and Atrial Capture Management™ (VCM and ACM) and Anti-Tachycardia Pacing (ATP) to eliminate manual threshold checks and extend longevity of the device by up to one year.ii Diagnostics, such as the Cardiac Compass® Report, and AF management tools that assist physicians in the early detection and treatment of atrial fibrillation. Remote monitoring via Medtronic’s CareLink® Network, which transmits comprehensive arrhythmia and diagnostic device data to a physician’s clinic. Rate Drop Response that identifies abrupt cardiac slowing and responds by pacing the heart at an elevated rate, which may reduce the frequency of syncope (fainting) in patients with apparent cardio-inhibitory vasovagal syncope.iii, iv High upper tracking rate to provide pacing support at higher heart rates for active and younger patients. The implanted pacing system must consist solely of an Advisa MRI device and two CapSureFix MRI™ SureScan Model 5086MRI leads, which must be used together. Leads are insulated wires that carry precisely timed electrical impulses from the pacemaker in the patient’s chest to a specific point on the inner heart wall. MRI Overview and Facts MRI scans allow physicians to make a wide range of health diagnoses by viewing highly detailed images of internal organs, blood vessels, muscles, joints, tumors, areas of infection and more. The number of MRI scans performed increases each yearv, as does the number of people with implanted cardiac devices.vi Until Medtronic’s firstgeneration Revo MRI® SureScan® pacing system, which was the only FDA approved (February 2011) MR-Conditional pacemaker in the U.S. before the Advisa MRI system, MRI procedures were not recommended in the U.S. for patients who have implanted pacemakers. Approximately 60 million MRI procedures are performed worldwide each year.vii In 2007, there were approximately 30 million MRI scans performed in the U.S., and its use continues to grow.viii Each year, approximately 320,000 people in the U.S. are implanted with a pacemaker.ix 10 percent of Revo patients are receiving an MRI at 18 months post implantx It has been estimated that there is a 50 to 75 percent probability that cardiac device patients will be indicated for an MRI over the lifetime of their devices. xi An estimated 200,000 patients in the U.S. annually have to forego an MRI scan because they have a pacemaker.xii More than 100,000 Medtronic SureScan devices have been sold worldwide;xiii and, in the U.S., more than 2,700 patients with SureScan pacing systems have received MRIs.xiv About the MR Classification System The American Society for Testing and Materials (ASTM) is an international standards organization that has developed a classification system for implanted and ancillary clinical devices: MR Safe: An item that poses no known hazards in all MR environments. (This would be something that contains no metal or any other type of electrical conducting surface.) MR Conditional: An item that has been demonstrated to pose no known hazards in a specific MR environment with specific conditions of use. (e.g., Revo MRI) MR Unsafe: An item that is known to pose hazards in all MR environments. Advisa MRI Clinical Trial The Advisa MRI Clinical Trial began with the first enrollment in June 2010. This confirmatory clinical trial of MRI use for pacemaker patients was conducted at 35 centers in the United States, Canada, Australia, Europe and the Middle East; 263 patients were implanted with a complete pacing system. The clinical trial was a prospective, randomized, controlled, non-blinded, multi-center investigational trial. Randomization of subjects to control and MRI groups was used to provide treatment effect information. The primary endpoints included: Safety: to assess the one month post-scan MRI-related complications. Effectiveness: to compare the changes in 1) atrial and 2) ventricular pacing capture thresholds before and one month after MRI between the MRI and control groups. The results of the confirmatory clinical study showed that all primary safety and effectiveness endpoints were met. Overall, there was no difference in performance between the MRI group and the control group.xv Advisa MRI received CE Mark approval in June 2009. ### i Gillis AM, Pürerfellner H, Israel CW, et al. Reduction of unnecessary ventricular pacing due to the Managed Ventricular Pacing (MVP) mode in pacemaker patients: Benefit for both sinus node disease (SND) and AV block (AVB) indications. Heart Rhythm. May 2005;2(5):S40. Abstract AB21-1. ii Rosenthal LS, Mester S, Rakovec P, et al. Factors influencing pacemaker generator longevity: results from the complete automatic pacing threshold utilization recorded in the CAPTURE Trial. Pacing Clin Electrophysiol. August 2010;33(8):1020-1030. iii Nordlander R, Hedman A, Phersson SK. Rate responsive pacing and exercise capacity—a comment. PACE. 1989;12:749-751. iv Stone J, Crossley G. Current sensor technology for heart rate modulation by artificial pacing. Clinical Electrophysiology Review. 1999;3:10-14. v IMV, “Benchmark Report: MRI 2007,” IMV Medical Information Division. Des Plaines, IL. 2008. vi Zhan C, Baine WB, Sedrakyan , A, et al. Cardiac device implantation in the United States from 1997 through 2004: A population-based analysis. J Gen Intern Med 2008; 23(Suppl 1): 13–19. vii Sutton R, Kanal E, Wilkoff BL, Bello D, et al. Safety of magnetic resonance imaging of patients with a new Medtronic EnRhythm MRI SureScan pacing system: clinical study design. Trials 2008, 9:68 viii IMV, “Benchmark Report: MRI 2007,” IMV Medical Information Division. Des Plaines, IL. 2008. ix Zhan C, Baine WB, Sedrakyan , A, et al. Cardiac device implantation in the United States from 1997 through 2004: A population-based analysis. J Gen Intern Med 2008; 23(Suppl 1): 13–19. x Medtronic Data on File, Nov 5, 2012 xi Kalin R and Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. PACE 2005;28:326-328. xii Medtronic calculations cited in Rod Gimbel and Ted McKenna, “Safety of Implantable Pacemakers and Cardioverter Defibrillators in the Magnetic Resonance Imaging Environment,” Business Briefing: Long-Term Healthcare 2005 (2005) available at www.touchbriefings.com. xiii Medtronic data on file. November 27, 2012. xiv Medtronic data on file. November 5, 2012. xv ADVISA DR MRI™ SURESCAN® PACING SYSTEM CLINICAL STUDY: Summary of clinical results