* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download General Physics (PHY 2130) - Wayne State University Physics and
Survey
Document related concepts
Transcript
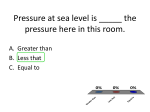
General Physics (PHY 2130) Lecture 22 • Solids and fluids density and pressure Pascal‘s principle and car lift http://www.physics.wayne.edu/~apetrov/PHY2130/ Lightning Review Last lecture: 1. Rotational dynamics torque and angular momentum two equillibrium conditions Review Problem: A figure skater stands on one spot on the ice (assumed frictionless) and spins around with her arms extended. When she pulls in her arms, she reduces her rotational inertia and her angular speed increases so that her angular momentum is conserved. Compared to her initial rotational kinetic energy, her rotational kinetic energy after she has pulled in her arms must be 1. the same. 2. larger because she’s rotating faster. 3. smaller because her rotational inertia is smaller. Example: Given: Moments of inertia: I1 and I2 Rotational kinetic energy is KErot = 1 2 1 Iω = Lω 2 2 We know that (a) angular momentum L is conserved and (b) angular velocity increases Find: K2 =? Thus, rotational kinetic energy must increase! Solids and Fluids Question What is a fluid? 1. 2. 3. 4. A liquid A gas Anything that flows Anything that can be made to change shape. States of matter: Phase Transitions ICE WATER Add heat STEAM Add heat These are three states of matter (plasma is another one) States of Matter • Solid Has definite volume Has definite shape Molecules are held in specific location by electrical forces and vibrate about equilibrium positions Can be modeled as springs connecting molecules • Liquid • Gas • Plasma States of Matter • Solid Crystalline solid Atoms have an ordered structure Example is salt (red spheres are Na+ ions, blue spheres represent Cl- ions) Amorphous Solid Atoms are arranged randomly Examples include glass • Liquid • Gas • Plasma States of Matter • Solid • Liquid Has a definite volume No definite shape Exist at a higher temperature than solids The molecules “wander” through the liquid in a random fashion The intermolecular forces are not strong enough to keep the molecules in a fixed position • Gas • Plasma States of Matter • Solid • Liquid • Gas • Plasma Has no definite volume Has no definite shape Molecules are in constant random motion The molecules exert only weak forces on each other Average distance between molecules is large compared to the size of the molecules States of Matter • Solid • Liquid • Gas • Plasma Matter heated to a very high temperature Many of the electrons are freed from the nucleus Result is a collection of free, electrically charged ions Plasmas exist inside stars or experimental reactors or fluorescent light bulbs! Is there a concept that helps to distinguish between those states of matter? Density • The density of a substance of uniform composition is defined as its mass per unit volume: m ρ= V some examples: 4 Vsphere = π R 3 3 Vcylinder = π R 2 h Vcube = a 3 • The densities of most liquids and solids vary slightly with changes in temperature and pressure • Densities of gases vary greatly with changes in temperature and pressure (and generally 1000 smaller) Units SI kg/m3 CGS g/cm3 (1 g/cm3=1000 kg/m3 ) 14 Fluids • Fluids: (liquids and gases) are materials that flow. • Fluids are easily deformable by external forces. • A liquid is incompressible. Its volume is fixed and is impossible to change. • A liquid will flow to take the shape of the container that holds it. A gas will completely fill its container. 15 Pressure Pressure arises from the collisions between the particles of a fluid with another object (container walls for example). There is a momentum change (impulse) that is away from the container walls. There must be a force exerted on the particle by the wall. By Newton’s 3rd Law, there is a force on the wall due to the particle. Pressure • Pressure of fluid is the ratio of the force exerted by a fluid on a submerged object or on the wall of the vessel to area F P≡ A Units SI Pascal (Pa=N/m2) Example: 100 N over 1 m2 is P=(100 N)/(1 m2)=100 N/m2=100 Pa. Often: 1 atmosphere (atm) = 101.3 kPa = 1.013 x 105 Pa 17 Example: Someone steps on your toe, exerting a force of 500 N on an area of 1.0 cm2. What is the average pressure on that area in atmospheres? First, let’s change centimeters into meters: 2 ⎛ 1 m ⎞ −4 2 1.0 cm 2 ⎜ ⎟ = 1.0 ×10 m ⎝ 100 cm ⎠ Then, using the definition of pressure, let’s determine pressure in Pa and then change to atm: F 500 N Pav = = A 1.0 × 10 − 4 m 2 1 atm ⎞ 6 2 ⎛ 1 Pa ⎞⎛ = 5.0 × 10 N/m ⎜ ⎟ 2 ⎟⎜ 5 ⎝ 1 N/m ⎠⎝ 1.013 × 10 Pa ⎠ = 49 atm Pressure and Depth • If a fluid is at rest in a container, all portions of the fluid must be in static equilibrium • All points at the same depth must be at the same pressure (otherwise, the fluid would not be in equilibrium) • Three external forces act on the region of a cross-sectional area A External forces: atmospheric, weight, normal ∑F = 0 ⇒ PA − Mg − P0 A = 0, but : M = ρ V = ρ Ah, so : PA = P0 A + ρ Agh P = P0 + ρ gh ConcepTest 1 You are measuring the pressure at the depth of 10 cm in three different containers. Rank the values of pressure from the greatest to the smallest: 1. 2. 3. 4. 1-2-3 2-1-3 3-2-1 It’s the same in all three 10 cm 1 2 3 ConcepTest 1 You are measuring the pressure at the depth of 10 cm in three different containers. Rank the values of pressure from the greatest to the smallest: 1. 2. 3. 4. 1-2-3 2-1-3 3-2-1 It’s the same in all three 10 cm 1 2 3 Pressure and Depth equation P = Po + ρgh • Po is normal atmospheric pressure • 1.013 x 105 Pa = 14.7 lb/ in2 • The pressure does not depend upon the shape of the container Other units of pressure: 76.0 cm of mercury One atmosphere 1 atm = 1.013 x 105 Pa 14.7 lb/in2 Example: Find pressure at 100 m below ocean surface. Given: masses: h=100 m P = P0 + ρ H 2O gh, so ( )( ) P = 9.8 ×105 Pa + 103 kg m3 9.8 m s 2 (100 m) ≈ 10 6 Pa Find: P=? (10 × atmospheric pressure) Pascal’s Principle • A change in pressure applied to an enclosed fluid is transmitted undiminished to every point of the fluid and to the walls of the container. • The hydraulic press is an important application of Pascal’s Principle F1 F2 P= = A1 A2 • Also used in hydraulic brakes, forklifts, car lifts, etc. Since A2>A1, then F2>F1 !!! 24 ΔP at point 1 = ΔP at point 2 F1 F2 = A1 A 2 ⎛ A 2 ⎞ ⎟⎟ F1 F2 = ⎜⎜ ⎝ A1 ⎠ The work done pressing the smaller piston (#1) equals the work done by the larger piston (#2). F1d1 = F2d2 Using an hydraulic lift reduces the amount of force needed to lift a load, but the work done is the same. 25 Example: Assume that a force of 500 N (about 110 lbs) is applied to the smaller piston in the previous figure. For each case, compute the force on the larger piston if the ratio of the piston areas (A2/A1) are 1, 10, and 100. Using Pascal’s Principle: A2 A1 1 10 100 F2 500 N 5000 N 50,000 N 26 Example: In the previous example, for the case A2/A1 = 10, it was found that F2/F1 = 10. If the larger piston needs to rise by 1 m, how far must the smaller piston be depressed? Since the work done by both pistons is the same, F2 d1 = d 2 = 10 m F1 27 Example: Depressing the brake pedal in a car pushes on a piston with crosssectional area 3.0 cm2. The piston applies pressure to the brake fluid, which is connected to two pistons, each with area 12.0 cm2. Each of these pistons presses a brake pad against one side of a rotor attached to one of the rotating wheels. See the figure for this problem. (a) When the force applied by the brake pedal to the small piston is 7.5 N, what is the normal force applied to each side of the rotor? The pressure in the fluid Also, Pr essure P = P = Fb Ab . Normal Force N Area of the brake pad piston A the normal force applied to each side of the rotor A 12.0 cm 2 N = PA = Fb = (7.5 N) = 30 N 2 Ab 3.0 cm