* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Phylum Cnidaria
Survey
Document related concepts
Transcript
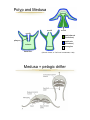
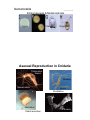
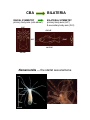
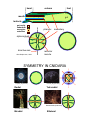
Lecture 2 Phylum Cnidaria Anemones, corals, jellyfishes, hydras and relatives by John R. Finnerty Animal Phylogeny Calcispongia Silicispongiae Porifera Ctenophora Cnidaria Deuterostomia Ecdysozoa Chordata Arthropoda Hemichordata Onychophora Echinodermata Nematoda Acoelomorpha Lophotrochozoa Annelida Mollusca Platyhelminthes PROTOSTOMIA Phylum Cnidaria Cnidaria (Greek: “stinging thread”) distinguished by the possession of cnidae 10,000 described species—sea anemones, corals, jellyfishes and hydras diploblast = 2 germ layers (ectoderm & endoderm) blind gut (single opening) nerve net & muscle cells radial symmetry??? sexual & asexual reproduction Cnidocytes & Cnidae Diagnostic of cnidarians. However, both ctenophores (Haeckelia [=Euchlora]) and aeolid nudibranchs may re-deploy cnidae that they have obtained from their cnidarian prey. (Barnes, Invertebrate Zoology, 1987) Cnidocytes & Cnidae The cnidocyte is a sensory-effector cell containing a cnida. Each cnida is a rounded proteinaceous capsule, with an opening on the apical surface that is often covered by a hinged operculum. At the surface, where the cnida opens, there are generally found a number of modified cilia, called cnidocil, which assist in the perception of tactile stimulation and chemical stimulation. In this sac, there is a long hollow thread. Upon mechanical contact and receipt of appropriate chemical stimuli, this thread is explosively everted from the sac. The cnidae may serve to deliver venom, like a hypodermic needle. Many “nematocysts” function like this. The cnidae may also serve to anchor the animal to a substrate or to adhere to a prey item. This adherent role can be performed by various subtypes of nematocysts but also by spirocysts and ptychocysts. Cnidae (Barnes, Invertebrate Zoology, 1987) (Pechenik, Biology of the Invertebates, 2000) Cnidae Discharge cytoplasm of cnidocyte cnida macromolecule (e.g., protein) Ca2+ cnida H2O stimulation calcium release increase in osmotic pressure water rushes in by osmosis cnida eversion of tubule Diploblasty Cnidaria diverged from Bilateria prior to the evolution of Mesoderm. So, Cnidaria lack mesoderm. Cnidaria are diploblasts, having only two germ layers, the primary germ layers: ectoderm and endoderm. Outer ectodermal epithelium (ectoderm) Inner gastrodermal epithelium (endoderm) Central layer of mesoglea of varying thickness. Mesoglea is a gelatinous, largely acellular substance. It may have a few living cells within it— often mobile amoeboid cells. However, the cells are not organized into a tissue like true mesoderm. The mesoglea can act as a hydrostatic skeleton, providing support to the rest of the cnidarian body which is really just two thin layers of epithelium Diploblasty mouth gastrodermis (endoderm) pharynx enteron epidermis (ectoderm) mesoglea basal disc “For those contemplating reincarnation, a major drawback to life as a cnidarian would seem to be the absence of an anus. All undigested food material passes through the same opening through which the food enters: the mouth. This is not particularly appetizing from the human point of view, but the shortcomings of life without an anus are not merely aesthetic. The sequential disassembly of particulate food material that occurs in an open-ended tubular gut is not possible in the cnidarian digestive system and, indeed, the animal must expel the undigested remains of one meal before it can ingest more food.” — Pechenik, 2001 In the through gut, different functions are localized to different sections of a linear tube. For example, consider your own digestive tract. Mouth Stomach Small Bowel Mechanical processing Protein hydrolysis Protein hydrolysis Initial carbohydrate digestion Pepsin (pH < 6) Trypsin (pH 7-9) Large Bowel Water resorption Carbohydrate digestion One-way gut In the one way gut, it is widely thought that you cannot have specialized regions of extracellular digestion. In other words, all extracellular digestion would have to occur in the same physio-chemical environment. In animals with one-way guts, there tends to be a greater emphasis on intracellular digestion, where undigested food particles are phagocytosed into the cells lining the gut. However, in both Cnidaria and Ctenophora, there is evidence for distinct gut regions with distinct extracellular environments Polyp and Medusa enteron mouth mouth mouth gastrodermis (endoderm) pharynx enteron basal disc epidermis (ectoderm) enteron mesoglea (Oliver & Coates, in The Fossil Invertebrates, 1992) Medusa = pelagic drifter Jellyfish are mixing the oceans? A theoretical model for the relative contributions of Darwinian mixing and turbulent wake mixing is created and validated by in situ field measurements of swimming jellyfish using a newly developed scuba-based laser velocimetry device. Extrapolation of these results to other animals is straightforward given knowledge of the animal shape and orientation during vertical migration. On the basis of calculations of a broad range of aquatic animal species, we conclude that biogenic mixing via Darwin’s mechanism can be a significant contributor to ocean mixing and nutrient transport. Polyp = sessile benthic (but capable of some movement) Cnidarian Nerve Net Cnidarian Nerve Net Spread of Excitation in Cnidarian Nerve Net Cellular Composition Cnidarian Muscle Histology Scale bar = 1.0 µm (Blanquet and Riordan, 1981) Cnidarian Diversity & Evolution ANTHOZOA — polyp body form only; simpler life histories; bilateral and biradial symmetry Class Anthozoa: sea anemones, corals, sea pens, etc. MEDUSOZOA — most have both polyp and medusa; radial and tetraradial symmetry Class Cubozoa: box jellyfishes Class Scyphozoa: true jellyfishes Class Hydrozoa: hydras, hydroids, hydromedusae Hydrozoan Life-History & Bodyplan Diversity Polyp Planula Medusa Colony Worm _Anthomedusae yes yes yes yes no _Leptomedusae yes yes yes yes no _Limnomedusae reduced yes yes no no _Trachymedusa no e _Hydra yes yes yes no no no no no no _Siphonophora yes yes yes no _Buddenbrockia no yes no yes yes _Myxobolus yes no yes yes yes no Cnidarian Phylogeny Anthozoa Cubozoa Scyphozoa Other Trachyline hydrozoa hydras hydrozoa - + - MEDUSOZOA (Bridge et al. 1997) Sexual Reproduction (Anthozoa) Broadcast spawning Planula larva Polyps Sexual Reproduction (Medusozoa) Medusae Broadcast spawning Planula larva Polyps Nematostella Embryogenesis & Metamorphosis Zygote Planula Polyp Asexual Reproduction in Cnidaria Transverse fission Budding Nematostella Metridium Pedal laceration Hydra Strobilation Aurelia “Cnidarians are radially symmetrical animals.” -Audesirk et al., 2001 -Barnes et al., 2001 -Brusca & Brusca, 1990 -Campbell et al., 2002 -Enger & Ross, 2003 -Lewis et al., 2004 -Mader, 2004 Hydra is radially symmetrical ectoderm endoderm colenteron (gut) mesoglea CBA RADIAL SYMMETRY primary body axis (oral-aboral) oral BILATERIA BILATERAL SYMMETRY primary body axis (A-P) & secondary body axis (D-V) dorsal ant. post. ventral aboral Nematostella — the starlet sea anemone oral Head Column Foot aboral head column foot mesentery mouth pharynx gut tentacle gut cavity endoderm mesoglea ectoderm siphonoglyph directive axis after Stephenson, 1926 pharynx mesentery * retractor muscle SYMMETRY IN CNIDARIA Porpita (Hydrozoa) Radial Aurelia (Scyphozoa) Tetraradial Nematostella (Anthozoa) Cerianthus (Anthozoa) Biradial Bilateral Why did bilateral symmetry originate? What was its original selective advantage? The Standard Explanation: Directed Locomotion An alternate scenario…. In the Cnidaria, locomotion is not correlated with symmetry. Modern Cnidaria are either sessile, or they locomote in a manner that is random with respect to their secondary axis. The bilaterally symmetrical corals and anemones are essentially sessile. The ancestral Cnidarian was a sessile polypoid animal. (Bridge et al., 1992, 1995, Collins 2003, and others) Therefore, bilateral symmetry did not evolve under selection for directed locomotion in the Cnidaria. In the Cnidaria, symmetry IS correlated with internal ciliary circulation…... Why is a sessile organism bilateral? pharynx mesentery siphonoglyph ciliary filaments on asulcal septum coelenteron Alcyonaria polyp Modified from Kaestner, 1984 An alternate scenario…. The correlation of symmetry with internal circulation holds for bilaterally symmetrical forms, bi-radially symmetrical forms, and tetradially symmetrical forms. Among polyps, true radiality characterizes the smallest hydrozoan polyps. Size interacts with symmetry and the location of ciliary tracts to affect the efficiency of internal circulation. Size dependency of internal polyp anatomy Anthozoa biradial or bilateral Scyphozoa tetraradial Hydrozoa radial biradial Can this selective explanation be extrapolated back to the CnidarianBilaterian Ancestor? If we assume: Homology of bilateral symmetry in Bilateria and Cnidaria (Finnerty et al., 2004) That the Cnidarian-Bilaterian ancestor was a sessile benthic animal (e.g., Collins, 2004). An alternate scenario…. Ancestral Bilaterian Ancestral Cnidarian Benthic Sessile Bilateral symmetry (manifest primarily internally) gut gut gut Benthic Crawling Bilateral symmetry (manifest internally & externally) ! Pronounced external manifestations of bilateral symmetry ! Centralized nervous system ! Directed locomotion. Cnidarian-Bilaterian Ancestor Bilateral symmetry (developmentally plastic?) Hox genes, dpp Finnerty, BioEssays 2005 Predictions and Implications… The location of ciliary tracts was under the control of “dorsalventral patterning genes” in the Cnidarian-Bilaterian ancestor. Perhaps this aspect of developmental gene regulation is conserved among modern Cnidaria and Bilateria. Variation in the arrangement of ciliary tracts within Cnidaria may be attributable to variation in the expression of dpp and other genes that pattern the “directive” axis.