* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Numerical Ages 1 -
Survey
Document related concepts
Transcript
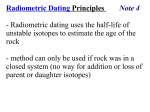
Absolute Time Part 1 -- Radioactivity Unless otherwise noted the artwork and photographs in this slide show are original and © by Burt Carter. Permission is granted to use them for non-commercial, non-profit educational purposes provided that credit is given for their origin. Permission is not granted for any commercial or for-profit use, including use at for-profit educational facilities. Other copyrighted material is used under the fair use clause of the copyright law of the United States. What to Look For: • Certain configurations of atomic nuclei are inherently unstable. We call atoms that have these configurations radioactive isotopes. • These atoms are subject to spontaneous “decay” which stabilizes the structure, either in one or several steps. • beta decay occurs when a neutron expels an electron, leaving it (+), and therefore a proton. This raises the atomic number by 1 but doesn’t change the atomic mass. (14C to 14N) • electron capture decay is the opposite. A proton absorbs an electron and becomes a neutron. This lowers the atomic number without changing the atomic mass. (14N to 14C) • alpha decay occurs when the nucleus emits 2 protons and 2 neutrons – a helium nucleus or alpha particle. (U to Pb involves several of these, as well as some betas). • The half-lives of even very long-lived isotopes can be determined by calculation from their easily determined decay constants. When Earnest Rutherford received the Nobel prize it was not just for working out the amount of heat energy released by radioactive elements. It was for working out the mechanism of that heat release and its consequences and implications. Not only did that work “discover the additional heat source” supposedly postulated by Kelvin, it also discovered, at last, a type of natural clock which was “wound” at the appropriate time for determining the age of the Earth (unlike the “Nile Delta clock” discussed earlier) and which has not yet run down (unlike the Ocean Salinity clock). Furthermore there are many copies of this clock in the world, all of them wound at different, but known, times, so we can date many events other than the origin of Earth. Finally, various versions of the clock run at different rates, so we can date very old events with some clocks almost as precisely as we can young events with other clocks. Ernest Lord Rutherford. Photographer unknown. First let’s look at the ways natural clocks work, both those that are useful and those that are not. We will begin with linear clocks. One way people used to keep track of short (but not tiny) periods of time was by carefully burning candles. This system works if 1) you are not interested in very small blocks of time, and 2) you are only interested in fairly short blocks, or, alternately, 3) you are careful to light a new candle at the exact time the old one burns out, for as long as is necessary to measure a big block of time. This works because candles deliver fuel (wax) to the flame at a pretty predictable rate. The wax (similar to oil when melted) “wicks” up the “wick” because of its surface tension and cohesion. Because the properties of molten wax and candle wicks are pretty consistent, the rate of delivery and therefore the burn rate is fairly constant. This is a “linear” process because if you graph the candle length against the time that it has been burning the result is a straight line. In this diagram we have used “half-lives” as the unit of time, for reasons that will become obvious. If the entire candle will burn for, say, two hours, the “half-life” is one hour. Note the straight line (dotted) that describes the burn rate. Both the accumulation of sediment on the Nile Delta and the accumulation of salt in the ocean were assumed to be linear, and this is probably not too bad an assumption. (Until the sediment source is worn down.) Notice that after two half-lives the candle is gone, as the ocean was “full” of salt. The clock runs down and afterwards you can’t tell when it did. Of course a candle can be marked off into however many segments you wish if you have a good ruler. Photograph by Thomas H. Johnson, Jr. Not all burning is linear, however. You are probably familiar with how open fires work. Once burning, with a good load of wood, they blaze bright and hot, but if you don’t add more wood periodically they “burn down”. They way a fire burns down is “non-linear” because the fuel is not delivered at a steady rate to the flame. There is, over time, less fuel available, so the fire “burns down” over time. As long as you sit beside the fire this is obvious and you can add more wood to keep the flame the size you like, but once you crawl into the tent the fire inevitably goes out. But the rate at which it goes out slows, so if you’re lucky there will still be hot coals in the morning to restart it. The graph shows the rate of wood consumption of a typical fire. early on the fire burns the fuel rapidly, producing a lot of heat and light, but this rate slows, predictably, and the heat/light output diminishes as a result. The red lines point out that half the wood is consumed in far less than half the total burn time. That is, there are more than two half-lives in the process. It would seem that a non-linear process like an open fire might make a better clock than a candle because you wouldn’t have to light a new one as often. However, there are too many variables for this to be accurate. Different types of wood burn at different rates, different sizes of wood from the same tree burn at different rates because of differences in surface area and volume. And whereas it is easy to make a mark half-way down a candle, it is essentially impossible to tell when exactly half a stack of wood has burned away. Most of the product goes up in smoke, literally. Still, if you’ve seen enough old cowboy movies you know that posse scouts use fires as rough clocks. A good one can tell how many hours ahead the bad guys are by how warm their fire still is. This works because oater bad guys, being bad guys, ignore Smokey Bear and never put out their campfires. Idiots. (Incidentally, if you don’t know that good posse scouts can do this then you need to go check out more cowboy movies. You’re Americans for crying out loud (or learning about American culture) and you should be familiar with cowboy mythology. Be sure you include “Shane”, some John Wayne (including the first “True Grit”), and the spaghetti westerns of Sergio Leone and Clint Eastwood in your viewing list.) The most important thing that Rutherford discovered about radioactivity (for our purposes in this class) was that it can be used as a non-linear clock that doesn’t have the same problems as an ordinary fire. Unlike an unfed fire (which will last a matter of hours or days) it runs for a long time (1000’s to over 10 billion years) in enough (but not all) cases to be useful. In addition it is comparatively easy to tell at any point in the process how far it has proceeded, if you have the right equipment, because all of the residue ordinarily remains and can be measured along with the unconsumed “fuel”. If this is not the case, it will be obvious. First we will look at the process and a bit of how Rutherford worked out its characteristics, then we’ll look at the machine and what can be done with it. Finally we will look at some critical tests of the hypothesis that it is reliable. (Actually the hypothesis will be that it is unreliable, but we will disprove it.) Consider first a “normal” atom of carbon. The other versions of carbon we will see are perfectly real and in no way “abnormal”, except that they do not have the simplest predictable nuclear structure. The atomic number of this atom is 6 (as in all carbon) because it has 6 (+) protons, represented here by purple balls. The atomic mass is 12 because there are 12 total mass units – 6 (+) and 6 (electrically neutral) neutrons (n). electron – e- neutron – (n) proton – (+) There are 6 electrons to balance the 6 protons in two shells – 2 in an inner and 4 in an outer shell. These are interesting che4mically, but not in terms of the nuclear reactions we will examine, so this is the last time we will show them. Remember that they are there even if we don’t show them in our diagrams. We’ve seen that “ordinary” carbon has an atomic mass of 12 because it has 12 massbearing nuclear particles. This version is designated 12C. Other versions of carbon exist, differing not in their number of protons (which is what makes them carbon in the first place) but in their mass -- the total number of nuclear particles. Because the proton number must be the same in any version of carbon, the difference is therefore the number of neutrons. One fairly uncommon version has one additional neutron and is therefore 13C. The version we will examine has two additional neutrons and is therefore 14C. Different mass versions of the same element are called isotopes. 14C is a radioactive isotope. This means it is unstable and has a high probability of being modified by a process we call radioactive decay. In the first geology course we usually introduce protons as particles with no charge, but certain types of nuclear reactions indicate that this is not exactly true. In such reactions either a neutron can be given a positive charge (making it a proton) or a proton can be neutralized (making it a neutron). Both reactions also involve a small, massless particle of charge (-1). This of course is an electron. So a neutron has the same mass as a proton because it is a proton, but with it’s charge neutralized by a contained electron. a neutron is … (+) neu a neutralized proton (-) There are three common ways that a radioactive isotope decays. 14C does it in a way called beta decay. beta (= e-) 14C One of the neutrons spontaneously (and randomly) emits a beta particle which is, in effect, an electron. As a result, that neutron is no longer neutral, but positive. Recall that an electron has effectively no mass. The particle that remains in the nucleus therefore has the same mass as ever, but is now positive. In other words it is a proton. This means that the atom now has 7 rather than 6 protons. (The outer e- shell will add an electron to balance this charge.) An atom with 7 protons (atomic number 7) is not carbon, but rather nitrogen. One element has transformed into another – 14C is now 14N! 14N In this instance C is the parent and N the daughter isotope. The atmosphere is mostly nitrogen and in its upper part (the ionosphere) the N atoms are constantly bombarded by and are “absorbing” ionized particles from the sun. This is the solar wind. and it doesn’t reach the surface (and wreck living tissue) partly because of this process of “absorption” in the ionosphere. e14N Some of the solar wind particles are electrons and they can interact with 14N in such a way that the previous process is reversed – a proton “absorbs” an electron, neutralizing its positive charge and making it a neutron. This is electron capture. Now the atom has 6 protons and 8 neutrons so it is no longer 14N but has changed to 14C. Electron capture in this case has created a radioactive atom that is now subject to beta decay, and so can produce 14N again! This ability to undo decay and recreate the original atom, then cycle through another decay is possible in some radioactive isotopes but not all. We will come back to this point later as we discuss tests of the hypothesis of radioactive dating. 14C For the final type of decay we will use a hypothetical atom of 6 protons to show the process, but be warned – carbon atoms themselves do not experience this type of decay. I use a small atom schematically to avoid having to draw (and have you count the particles in) a very large one. HYPOTHETICAL SMALL PARENT ATOM THAT IS NOT CARBON!!!! In the final decay type a very large nucleus may eject four mass particles – two each of protons and neutrons – as a unit. this is effectively a helium (4He) nucleus. These large particles are potentially very damaging and carry a lot of energy. This is where most of the heat that Rutherford discovered comes from. Alpha decay leaves the parent isotope 4 mass units lighter and two atomic numbers lower. If carbon did decay this way (it does not – don’t forget it) the daughter isotope would have atomic number 4 and atomic mass 8. It would be 8Be in other words. 4He HYPOTHETICAL SMALL DAUGHTER ATOM THAT IS NOT BERYLLIUM!!!! nucleus (or “alpha particle”) (or “alpha ray”) emitted There are a fairly large number of radioactive isotopes in the universe, but for now we are only interested in a small percentage of them. Some of the larger atoms (like certain Uranium (U) isotopes) do not decay directly to their daughter (a lead (Pb) isotope in the case of U), but do so through a series of alpha, beta, e- capture steps. Whereas the total pathway from U to Pb has an exceptionally long half-life, the intermediates have much shorter ones. They decay at rates measurable in realtime in a laboratory. It was by studying these decay events that Rutherford discovered the principle of the decay constant. This is an easily determined value from which can be calculated the half life of even very long-lived isotopes. This illustrates very nicely an idea we presented on day 1 – how to know something. We know the half-lives of many short-lived isotopes from direct observation. We know that the decay constant in each case can be used to predict that half-life. Therefore we “know” that the decay constant of U or some other long-lived isotope can be predicted from its decay constant. That is, there is an intermediate (but mathematically provable) intermediate step required to know what the half-life of a long-lived isotope is. We do not exist, either individually or collectively, for long enough to measure it directly. Take-Home Message • Certain configurations of atomic nuclei are inherently unstable. We call atoms that have these configurations radioactive isotopes. • These atoms are subject to spontaneous “decay” which stabilizes the structure, either in one or several steps. • beta decay occurs when a neutron expels an electron, leaving it (+), and therefore a proton. This raises the atomic number by 1 but doesn’t change the atomic mass. (14C to 14N) • electron capture is the opposite. A proton absorbs an electron and becomes a neutron. This lowers the atomic number without changing the atomic mass. (14N to 14C) • alpha decay occurs when the nucleus emits 2 protons and 2 neutrons – a helium nucleus or alpha particle. (U to Pb involves several of these, as well as some betas). • The half-lives of even very long-lived isotopes can be determined by calculation from their easily determined decay constants.