* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download JACC-HF Online Appendix 1- Study Oversight
Baker Heart and Diabetes Institute wikipedia , lookup
Electrocardiography wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Heart failure wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
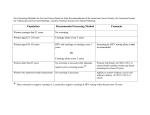
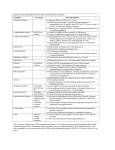
CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. Online Appendix 1- Study Oversight CUPID Clinical Endpoints Committee (CEC) Responsibilities: The CEC provides independent, consistent and unbiased adjudication of all potential of protocoldefined study events using clearly defined standardized criteria for heart failure hospitalization. The CEC also evaluates whether the decisions and timing regarding LVAD and heart transplant are in accord with current heart failure practice guidelines. This committee will remain blinded to treatment assignment throughout the study. CEC Committee Chairman: Akshay Desai M.D., MPH, Brigham and Women’s Hospital Clinical Endpoint Center CUPID Data Monitoring Committee (DMC) Responsibilities: The DMC is responsible for safeguarding the interests of trial participants, assessing the safety and efficacy data during the trial, monitoring the overall conduct of the clinical trial and providing recommendations for enhancing the integrity of the trial. Members and affiliations: Jeffrey S. Borer, M.D., Chair, Professor of Medicine, Cell Biology, Radiology and Surgery Chief, Division of Cardiovascular Medicine, Director, The Howard Gilman Institute for Heart Valve Diseases and the 7 January 2014 JACC-HF-1 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. Schiavone Cardiovascular Translational Research Institute State University of New York Downstate Medical Center, New York, NY Lloyd Fisher, M.D., Ph.D., Professor Emeritus, Department of Biostatistics, University of Washington, Kenmore, WA Alan B. Miller, M.D., Professor of Medicine, Division of Cardiology, University of Florida Health Science Center, Jacksonville, FL Ian Sarembock, M.D., Ch.B., M.B., Service Line Executive Medical Director & Co-Director, Heart & Vascular Center at The Christ Hospital, Cincinnati, OH Karl Swedberg, M.D., Ph.D., Senior Physician, Department of Medicine, Sahlgrenska University Hospital-Östra, Göteborg, Sweden CUPID Executive Steering Committee Responsibilities: The Executive Steering Committee is responsible for overseeing the conduct of the clinical trial, to provide information, insight and feedback to the DMC and CEC and to provide medical and scientific advice on all aspects of this study and other issues that arise during the clinical development of AAV1/SERCA2a. Members and affiliations: Barry Greenberg, M.D. (Chair); Professor of Medicine, Director, Advanced Heart Failure Treatment Program, University of California, San Diego, San Diego CA 7 January 2014 JACC-HF-2 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. Javed Butler, M.D., MPH, FACC, FAHA, Professor of Medicine, Division of Cardiology, Director, Heart Failure Research, Emory University School of Medicine, Atlanta, GA G. Michael Felker, M.D., MHS, Associate Professor of Medicine, Division of Cardiovascular Medicine, Duke University, Durham, NC Adriaan Voors, M.D., Ph.D., Professor of Cardiology, University Medical Center Groningen, Groningen, The Netherlands 7 January 2014 JACC-HF-3 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. Online Tables Full Study Inclusion / Exclusion Criteria Online Table 1 Inclusion Criteria Unless otherwise specified, screening must be performed within 30 days prior to administration of investigational medicinal product on Day 0 except as noted under Inclusion Criteria #1 and 4. Subjects must meet the following criteria to be eligible for the study: 1. Negative neutralizing AAV1 antibodies (NAb) (titer <1:2 or equivocal) within 90 days of screening. 2. 18-80 years of age, inclusive, at the time of signing the informed consent. 3. Chronic systolic HF due to ischemic or non-ischemic cardiomyopathy. Subjects with ischemic cardiomyopathy must have at least one major coronary vessel with TIMI grade 3 flow. If a subject has not undergone coronary angiography within 2 months, this criterion may be assessed after the subject is randomized and undergoes angiography just prior to the planned infusion of investigational medicinal product. a. Hypertrophic cardiomyopathy is excluded. b. Toxic and alcoholic cardiomyopathies are allowed as long as toxin or alcohol exposure has been eliminated and a sufficient amount of time has elapsed to ruleout spontaneous recovery. 4. Left ventricular ejection fraction (LVEF) ≤35% anytime during the 60-day window prior to administration of investigational medicinal product. 5. Diagnosis of NYHA class II, III or IV HF for a minimum of 90 days prior to screening. 6. Individualized, maximal, optimized HF therapy consistent with American College of Cardiology/American Heart Association and European Society of Cardiology practice 7 January 2014 JACC-HF-4 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. guidelines for the treatment of chronic heart failure(ACC/AHA/ESC HF guidelines) and as updated from time to time: a. Medical therapy as appropriate to the individual subject including oral diuretic, angiotensin-converting enzyme (ACE) inhibitor (or angiotensin-receptor blocker (ARB) if ACE intolerant) and, as tolerated, beta blocker at approved dosages as labeled in the respective package insert. The choice of beta blocker is limited to those approved for heart failure in all participating countries (bisoprolol, carvedilol or sustained release metoprolol succinate). Unless contraindicated or not tolerated, the addition of an aldosterone antagonist should be considered in the absence of hyperkalemia and significant renal dysfunction and according to evolving standards; the final decision is at the discretion of the investigator. Dosing of the above medications must be stable for a minimum of 30 days prior to screening, although up- or down-titration of diuretics, as medically indicated, is permitted. Enrollment of any subject with any deviation from this combination must be preapproved by the medical monitor. b. Resynchronization therapy, if clinically indicated according to ACC/AHA/ESC HF guidelines, must have been implanted at least 6 months prior to screening. c. Implantable cardioverter defibrillator (ICD), if clinically indicated according to ACC/AHA/ESC HF guidelines, must have been implanted a minimum of 30 days prior to screening. d. Cardiac rehabilitation should be consistent with the Agency for Health Care Policy and Research Clinical Practice Guideline, Number 17, Cardiac Rehabilitation. This does not imply that the potential candidate must be enrolled 7 January 2014 JACC-HF-5 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. in a cardiac rehabilitation program at screening or in the future. 7. All women of childbearing potential must have a negative urine pregnancy test prior to administration of investigational medicinal product and agree to use adequate contraception (defined as oral or injectable contraceptives, intrauterine devices, surgical sterilization or a combination of a condom and spermicide) or limit sexual activity to vasectomized partner for 3 months after administration of investigational medicinal product. Men capable of fathering a child must agree to use barrier contraception (combination of a condom and spermicide) or limit activity to post-menopausal, surgically sterilized, or a contraception-practicing partner, for 3 months after administration of investigational medicinal product. 8. Ability to understand and comply with study requirements as evidenced by providing signed written informed consent form and Release of Medical Information Form. 9. Presence of at least one of the following risk factors: a. Hospitalization for heart failure within 6 months of screening, or in lieu of hospitalization, at least 2 outpatient interventions for the intended treatment of signs and symptoms of worsening heart failure (e.g., intravenous diuretics, peripheral ultrafiltration) b. NT-proBNP >1200 pg/mL (BNP >225 pg/mL) within 30 days of screening; if subject is in atrial fibrillation, NT-proBNP >1600 pg/mL (BNP >275 pg/mL) within 30 days of screening 10. In Germany only: Medically indicated for diagnostic angiography at the clinician’s discretion. 7 January 2014 JACC-HF-6 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. Online Table 2 Exclusion Criteria Subjects meeting any of the following criteria will be excluded from the study: 1. Any intravenous (IV) therapy with positive inotropes, vasodilators or diuretics within 30 days prior to screening. 2. Restrictive cardiomyopathy, obstructive cardiomyopathy, acute myocarditis, pericardial disease, amyloidosis, infiltrative cardiomyopathy, uncorrected thyroid disease or discrete LV aneurysm. 3. Cardiac surgery, percutaneous coronary intervention (PCI) or valvuloplasty within 30 days prior to screening. 4. Myocardial infarction (e.g., ST elevation MI [STEMI] or large non-STEMI) within 90 days prior to screening. Large non-STEMI shall be defined >3x ULN for CK-MB or >5x ULN for troponin. 5. Prior heart transplant, left ventricular reduction surgery (LVRS), cardiomyoplasty, passive restraint device (e.g., CorCap™ Cardiac Support Device, Acorn Cardiovascular Inc., St. Paul, MN), surgically implanted LVAD or cardiac shunt. 6. Likely need for an immediate heart transplant or LVAD implant due to hemodynamic instability. 7. Prior CABG is not considered ideal for inclusion in the study; however, a potential candidate can be reviewed on a case-by-case basis. Ideally, the orifice of the graft should be easy to engage with a catheter and the graft should perfuse a significant amount of potentially viable myocardium. 8. Known hypersensitivity to contrast agents used for angiography; history of, or likely need 7 January 2014 JACC-HF-7 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. for, high dose steroid pretreatment prior to contrast angiography. 9. Significant, in the opinion of the investigator, left main or ostial right coronary luminal stenosis. 10. Liver function tests (ALT, AST, alkaline phosphatase) >3x Upper Limit of Normal (ULN) within 30 days prior to investigational medicinal product administration or known intrinsic liver disease (e.g., cirrhosis, chronic hepatitis B or hepatitis C virus infection). 11. Current or likely need for hemodialysis within 12 months following enrollment or current GFR ≤20 mL/minute/1.73 m2 estimated by MDRD calculation. 12. Bleeding diathesis or thrombocytopenia defined as platelet count <50,000 platelets/μL. 13. Anemia defined as hemoglobin <9 g/dL, provided that there is no evidence of bleeding. 14. Known AIDS or HIV seropositive status, or a previous diagnosis of immunodeficiency with an absolute neutrophil count <1000 cells/mm3. 15. Diagnosis of, or treatment for, any cancer other than basal cell carcinoma within the last 5 years. (Past medical history of cancer is not exclusionary as long as subject has been disease-free for at least 5 years since the time of diagnosis and treatment). 16. Previous participation in a study of gene transfer; however, if the study was unblinded or documentation otherwise exists that the subject was randomized to the placebo control group and did not receive active gene transfer agent, the subject may be considered for this study. 17. Receiving investigational intervention or participating in another clinical study within 30 days or within 5 half-lives of the investigational drug administration prior to screening. Exception may be made if the individual is enrolled in a non-therapeutic observational study (registry) or the observational portion of a therapeutic study where the sponsoring authority authorizes enrollment. 7 January 2014 JACC-HF-8 CUPID 2: AAV1/SERCA2a in Heart Failure Greenberg, et al. 18. Pregnant or breast-feeding. 19. Recent history of psychiatric disease, including drug or alcohol abuse, that is likely to impair, in the opinion of the investigator, the subject’s ability to comply with protocol-mandated procedures. 20. Other concurrent medical condition(s) that, while not explicitly excluded by the protocol, could jeopardize the safety of the subject or objectives of the study. Table abbreviations: AIDS Acquired immunodeficiency syndrome ALT Alanine aminotransferase AST Aspartate aminotransferase BNP B-Type natriuretic peptide CABG Coronary artery bypass graft(s) HIV Human immunodeficiency virus LV Left ventricular or left ventricle MI Myocardial infarction NAb Neutralizing AAV1 antibodies NT-proBNP N-terminal pro B type natriuretic peptide TIMI Thrombolysis In Myocardial Infarction TIMI Grade Flow is a scoring system from 0-3 referring to levels of coronary blood flow STEMI ST segment elevation myocardial infarction ULN Upper Limit of Normal 7 January 2014 JACC-HF-9