* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Opiates: Good or Bad?
Drug design wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Urban legends about drugs wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
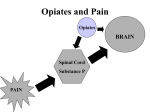
Opiates: Good or Bad? Mandeep Pabla, Julie Lee, Kelsey Lee A young woman pregnant with her first child makes a visit to her obstetrician. She imagines holding her baby as she sees the black and white sonogram. Moments later, she participates in a routine urine testing and is shocked to find out she tested positive for opiates. The atmosphere in the bright room suddenly dims as the obstetrician announces the results. Although the soon-to-be mother has never abused drugs, she immediately fears losing her child to Child Protection Services. The cause of her positive results for morphine and codeine was not heroin, but a poppy seed muffin she ate earlier for breakfast. Opiates are alkaloids found naturally in the seedpods of poppy plants, Papaver somniferum. Many obstetricians also require prenatal drug tests in order to ensure the health of the developing children. However, drug tests are unable to determine the source of the drug; whether the compound entered the body in the form of a poppy seed muffin or heroin. Some people who test positive for any type of opiates offer the excuse of eating a pastry with poppy seeds after a quick search online, without understanding how the compounds are metabolized in the body. Studies have found that heroin is metabolized in the body into morphine, and codeine is metabolized into morphine and hydrocodone [1]. Poppy seeds contain both codeine and morphine, and Figure 1: A generalized structure of opiates, codeine is metabolized at a slower rate than where X = -H, -CH3, or –COCH3 heroin. Therefore, someone who tests positive for morphine and negative for codeine is likely to have used opiates recreationally. These compounds partially desensitize the body by blocking some of the pain signals between the peripheral and central nervous systems [2,3]. Due to this mechanism, opiates are abused recreationally as street drugs. Addicts are unable to limit their intake because of the drugs creates false sense of euphoria. It is believed that opiates activate the dopaminergic systems, which leads to an opiate high, although it is not completely understood [4]. Due to the highly addictive nature of opiates, users are likely to solicit in illegal activities or become violent because of their desperation for the drug. Most US employers hold regular screenings for illegal drugs from employees to maintain a safe work environment. Despite the bad reputation of opiates, they are also used in medicine as effective pain relievers [5]. Popularly prescribed drugs include the brands Vicodin and OxyContin, which are typically used to treat chronic pain. In order to keep up with the pharmaceutical demand, methods of synthesizing opiates in were determined. They are characterized by the following structure, shown in Figure 1. Opiates can be consumed orally, injected directly into the bloodstream, snorted, or smoked. These compounds are soluble in aqueous environments due to the hydrophobic nature of the polycyclic hydrocarbon rings, so they can freely travel through the bloodstream. Opiates permeate across the blood brain barrier to interact with their receptors located in the nerves of the central nervous system. The four classes of opioid receptors are all rhodopsin G-protein coupled receptors (GPCRs) found on the descending inhibitory system for pain [6]. As GPCRs, opioid receptors are characterized with seven trans membrane domains and an extracellular glycosylated N terminus. The receptors vary the most in structure in the N and C termini, and the extracellular loops [6]. When opiates interact with their respective receptors, the membrane is hyperpolarized by the unregulated opening of potassium channels [5]. Morphine, codeine, and thebaine are opiates, and oxycodone is an opiate derivative derived from the natural opium alkaloid, thebaine. Many illicit users abuse oxycodone to reach a euphoric high, while prescribed users take this opioid analgesic medication to control the most severe pain. With oxycodone’s synthesis in the early 1900s, an alternative for morphine and codeine without the harmful side effects was discovered. The intention was that the thebaine-derived drug would have the analgesic effects of morphine and codeine without the euphoric effects that lead to abuse and addiction. Oxycodone shares many similarities with codeine. Not only is the chemical name, 14-hydroxy-7,8-dihydrocodeinone, derived from codeine, but also the structures are nearly identical. As seen in figures 1 and 2, one difference is the hydroxyl group of codeine oxidizes to a ketone in oxycodone, hence the suffix “-one”. Also, oxycodone has one less double bond between two carbons, and it has an impactful hydroxyl group at the 14’ C, while codeine has hydrogen at this position. The 14’ hydroxyl group makes the drug “50% more potent” than hydrocodone, which is a derivative of codeine [10]. Figure 2: Oxycodone, C18H21NO4 [7] Figure 3: Codeine, C18H21NO3 [8] Oxycodone is a primary ingredient in pain relief medications, such as Percocet and Tylox. A unique property of oxycodone is that its time released, meaning the effects occur over a set span of time versus all at once. The drug works through the central nervous system by changing pain messages traveling to the brain so the user’s sense of pain is altered. Oxycodone’s mechanism consists of “acting as an agonist at the mu, kappa, and delta opioid receptors within the central nervous system” [9]. When this opioid closes voltage-operated calcium channels (kappa receptor agonist) and opens calciumdependent potassium channels (mu and delta receptor agonist), hyperpolarization and neuronal excitability results [9]. Oxycodone is a Schedule II controlled substance with high abuse potential in the United States. The pain medications Percocet, Percodan, and Tylox contain oxycodone in small doses combined with other active ingredients. As an opiate, oxycodone is similar to heroin in its pleasurable effects; hence, it is misused easily. The drug elevates levels of dopamine, which is a neurotransmitter linked with euphoric experiences. Abusers crush the tablets, and ingest the powder orally, intranasally, or rectally. An underground illicit market has emerged due to the recreational benefits as prescribed patients sell their prescriptions for income. Opiates are difficult to synthesize due to the complex structures. Oxycodone is a semisynthetic opioid analgesic made from the precursor thebaine in opium poppy. The complete synthesis of oxycodone is “complicated and in low yield”; therefore, it is semisynthetically produced from thebaine [11]. Although the drug is highly regulated as a Schedule II controlled substance, this opioid is still widely prescribed to patients and can and is abused with habit-forming side effects. In 2009, “about four million young adults used prescription pain relievers on a recreational basis… hydrocodone, oxycodone, and sustained-released oxycodone have been the principle drugs involved [11]”. In the future, the development of a synthetic opioid that is not addictive and abusive is being considered. Another drug with high potential for abuse is hydrocodone, or Vicodin. Hydrocodone is a semi-synthetic opiate that is derived from 3-methylmorphine, or codeine [12]. It is a white, colorless drug that is usually taken orally as cough medicine or as a narcotic analgesic. According to the International Narcotics Control Board, 99% of the worldwide supply of hydrocodone was distributed within the United States [13]. Most users of hydrocodone feel a numbing sensation at high doses throughout the body and experience various withdrawals, such as severe pain and extreme anxiety. Figure 4: Hydrocodone, C18H21NO3 Structurally, although hydrocodone is derived from 3-methylmorphine, they are two completely different drugs. Codeine is the 3methylether of morphine and is not synthetic. It is inactive but can be activated by converting codeine into morphine in the presence of an enzyme called CYP2D6, which is located in the liver. CYP2D6 is an enzyme that is able to activate opioid effects. Both hydrocodone and codeine are derived from an opium poppy plant called Papaver Somniferum whereas codeine is found within the poppy pod. Since hydrocodone is a semi-synthetic, it is formed in the laboratory, but its makeup and constituents are found from the poppy pod as well [14]. Hydrocodone’s chemical structure is quite similar to codeine, but hydrocodone is different enough to make its effects much stronger and will lead to more drug abuse and overdose possibilities. Currently, research is being conducted on hydrocodone’s interaction in the body, but it is still relatively unknown as to which receptors hydrocodone binds to in the central nervous system. In the general sense, opiates relieve pain by interacting with the spinal cord, brain, and limbic system. Opiates prevent pain messengers from passing information between neurons to block these pain signals from reaching the brain. This results in major pain relief throughout the body. Opiates interact with various brain regions differently than how they interact with the spinal cord. Opiates block pain messengers and receptors in the spinal cord, but in the brain, they change the experience of pain. For example, when a patient takes hydrocodone for pain-relief purposes, he or she can experience or feel the pain, but it does not particularly bother or hurt them. The receptors in the brain responsible for relieving pain are called delta, mu, and kappa receptors [15]. Opiates thus block pain signals by binding to the mu receptor site in the brain. Lastly, opiates interact with the limbic system, typically known as the region of emotions. Opiates then produce feelings of relaxation, pleasure, and euphoria when being used by the patient. However, constant use of opiates leads to addiction and deterioration of the brain. Opiates alter the brain by causing side effects of anxiety, nausea, and many more. Over an extensive period of time, opiates can ruin cell communication in the brain by changing synapse and brain cells to a great extent that synapse information can be compulsive and erratic, completely changing a person’s personality [16]. REFERENCES: 1. Meadway C, George S, Braithwaite R. 1998. Opiate concentrations following the ingestion of poppy seed products—evidence for ‘the poppy seed defence.’ Forensic Science Internaional. 96: 29-38 2. Korf J, Bunney BS, Aghajanian GK. 1973. Noradrenergic neurons: morphine inhibition of spontaneous activity. European Journal of Pharmacology. 25: 165-9 3. Al-Hasani R, Bruchas MR. 2011. Molecular mechanisms of opioid receptordependent signaling and behavior. Anesthesiology. 115: 1363-81 4. Wise RA, Bozarth MA. 1985. Brain mechanisms of drug reward and euphoria. Psychiatr. Med. 3:445-60 5. North RA, Williams JT. 1983. How do opiates inhibit neurotransmitter release? TINS. 6: 337-9 6. Law PY, Wong YH, Loh HH. 2000. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 40: 389-430 7. CSID:4447649, http://www.chemspider.com/Chemical-Structure.4447649.html (accessed 07:15, Feb 13, 2014) 8. CSID:4447447, http://www.chemspider.com/Chemical-Structure.4447447.html (accessed 01:21, Feb 14, 2014) 9. "DrugBank: Open Data Drug & Drug Target Database.”. N.p., 13 Jun 2005. Web. 13 Feb 2014. <http://www.drugbank.ca/drugs/DB00497>. 10. "World of Molecules." Oxycodone--OxyContin. N.p.. Web. 13 Feb 2014. <http://www.worldofmolecules.com/drugs/oxycodone.htm>. 11. Levinthal, Charles. Drugs, Behavior, and Modern Society. Boston, MA: Pearson Education, 2012. Print. 12. Karch, Steven B. (2008). Pharmacokinetics and pharmacodynamics of abused drugs. Boca Raton: CRC Press. pp. 55–56. ISBN 1-4200-5458-9. 13. International Narcotics Control Board Report 2008.. United Nations Pubns. 2009. p. 20. ISBN 9211482321. 14. Hall, Christina. "What Is the Difference between Hydrocodone and Codeine?." . Conjecture Corporation, 9 2 2014. Web. 17 Feb 2014. <http://www.wisegeek.com/what-is-the-difference-between-hydrocodone-andcodeine.htm>. 15. "The Brain's Response to Opiates." Mind Over Matter. National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services, n.d. Web. 17 Feb 2014. <http://teens.drugabuse.gov/sites/default/files/opiates.pdf>. 16. Hagaman, Jennifer. "Opiates on the Brain." . N.p.. Web. 17 Feb 2014. <http://www.csulb.edu/~cwallis/483/opiates_on_the_brain.html>.