* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 11 Notes - Mr-Durands
Geothermal energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Solar thermal energy wikipedia , lookup
Alternative energy wikipedia , lookup
Solar water heating wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Gibbs free energy wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Geothermal heat pump wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
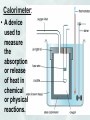
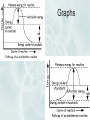
Good Morning 5/2/2017 • Today we will be working on the notes for ch 11. Chapter 11 Notes Thermochemistry: Investigates heat changes that occur during chemical reactions. Energy: • The ability to do work. Measured in Joules (J) or calories (cal) Work: • Force applied over distance. (W = F x d) work is also measured in joules Chemical Potential Energy: • Energy that is given off when chemical bonds are formed. Heat: Energy that is transferred from one substance to another. • Represented by a “q” or “H” in an equation. Heat is measured in Joules (J) or calories (cal.), the same units as energy. Heat flows from hot objects to cold objects. Only changes in heat can be detected. Law of Conservation of Energy: • In any chemical or physical process, energy is neither created nor destroyed. Most energy that is considered “lost” is usually just dispersed as heat into its surroundings. Exothermic: • Energy is released to its surroundings. Represented by the word “energy” in the products of a chemical equation. Heat flows out of the system. (Heat change < 0) Endothermic: • Energy is absorbed from its surroundings. Represented by the word “energy” in the reactants of a chemical equation. Heat flows into the system. (Heat change > 0) Heat Capacity: • The amount of heat needed to raise the temperature of an object exactly 1.0 C Specific Heat Capacity (Also called “Specific Heat”) The amount of energy needed to raise the temperature of 1 gram of the substance 1C. Represented by the symbol “C” in an equation. Specific Heat Capacity Label = J/gC Specific Heat Capacity Equation: q = H = m x T x C calorie: • English unit for measuring heat changes. The amount of energy needed to raise the temperature of 1 gram of Water by 1C. Calorie : • Equal to 1000 calories or 1 kcal. (Note the capital C) This unit is general used to measure the energy content in food. • It is what we mean when we talk abou the number of Calories in Food. Joule: • SI unit for measuring heat or energy. 1 Joule = energy needed to lift 1 N by 1 meter. 1 calorie = 4.184 Joules. kg m J N m 2 s 2 Calorimetry: • The accurate and precise measurement of heat changes for chemical or physical processes. Calorimeter: • A device used to measure the absorption or release of heat in chemical or physical reactions. Enthalpy (H): • The heat content of a system at constant pressure. Heat of Reaction: a.k.a. Enthalpy Change (ΔH) • The amount of heat absorbed or lost by a system during a process at constant temperature ΔH = H products – Hreactants ΔH is positive for an endothermic reactions (energy is gained) Heat content of products is greater than the heat content of the reactants ΔH is negative for an exothermic reactions (energy is lost) Graphs Heat of Combustion: • The heat released during a chemical reaction in which one mole of a substance is completely burned. Molar Heat of Fusion (Hfus): • The energy needed to melt 1 mole of a substance. Problem: The molar heat of fusion of water is 6.009 kJ/mol. How much energy is needed to convert 60 grams of ice at 0°C to liquid water at 0°C? 1 mol H2O 6.009 kJ 60g H2O x x = 20.00 kJ 18.02g H2O 1 mol H2O Molar Heat of Solidification (Hsolid): • The heat lost when 1 mole of a liquid solidifies. Molar Heat of Vaporization (Hvap): • The amount of heat necessary to vaporize 1 mole of a given liquid. Molar Heat of Condensation (Hcond): • The amount of heat released when 1 mole of a gas vapor condenses. Molar Heat of Solution (Hsoln): • The heat change that results when 1 mole of a substance is dissolved in water. Hess’s Law of Heat Summation: • If you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. Example: Eq 1 A + B D + C H = -175 J + Eq 2 CA+E H = 75 J = A + C + B D + E + A + C H = -100 J Writing Thermochemical Equations 1. Fraction coefficients may be used because coefficients represent mole quantities, NOT atoms or molecules 2. Use appropriate state/phase symbols (g) (l) (s) 3. ΔH is proportional to the number of moles 4. ΔH is usually not influenced significantly by the temperature of the system