* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Maintaining a balance
Organisms at high altitude wikipedia , lookup
Photosynthesis wikipedia , lookup
Human genetic resistance to malaria wikipedia , lookup
Environmental persistent pharmaceutical pollutant wikipedia , lookup
Hyperthermia wikipedia , lookup
List of types of proteins wikipedia , lookup
Natural environment wikipedia , lookup
Biochemistry wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Homeostasis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
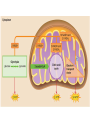
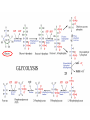
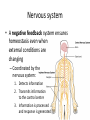
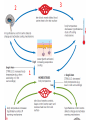
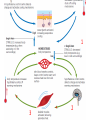
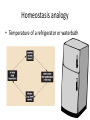
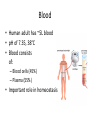
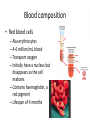
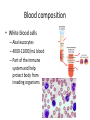
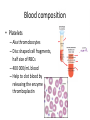
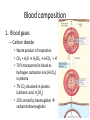
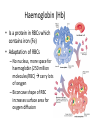
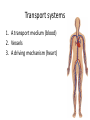
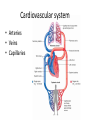
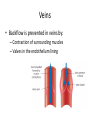
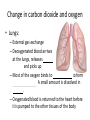
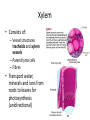
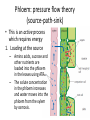
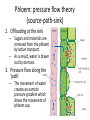
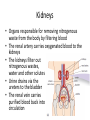
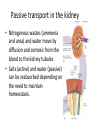
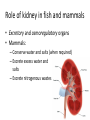
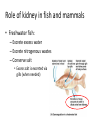
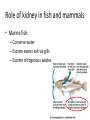
Maintaining a balance 1. Most organisms are active in a limited temperature range • identify the role of enzymes in metabolism, describe their chemical composition and use a simple model to describe their specificity on substrates Keeping things constant • Living organisms exist in a wide range of environmental conditions • Even so, organisms function best within a narrow range of temperatures • Organisms have adaptations that help to keep a relatively constant internal temperature and balance of chemicals – Homeostasis Keeping things constant • http://www.abc2news.com/sports/how-youcan-die-from-drinking-too-much-waterexplained Enzymes • Enzymes are important biological molecules that help maintain homeostasis • They also control the metabolic processes within the body – Metabolism: chemical reactions in the body • Catabolic reactions: breaking down of substances – Usually exergonic reactions (release energy) – e.g. breaking down of glucose to release energy • Anabolic reactions: building of larger molecules – Usually endergonic reactions (require energy) – e.g. production of proteins from amino acids Enzymes • Structure: globular proteins (cf fibrous and membrane) – Composed of a chain of amino acids which fold to form a 3-dimensional shape – (Somewhat) spherical – Soluble – Contain an active site Catalase Enzymes • Function: biological catalysts – Speed up the rate of metabolic reactions but are unchanged at the end of the reaction – Can be reused – Each enzyme can only catalyse one type of reaction because the active site recognises a specific molecule (substrate) Enzyme-substrate complex Enzymes • Reactions need activation energy to proceed • Enzymes function by lowering the activation energy required the reaction proceeds more quickly Lock and key model • The active site is fixed in shape • The shape of the substrate fits exactly into the active site like a lock and key • This forms the enzyme-substrate (ES) complex • When the reaction has taken place, the products are released from the active site Induced fit model • Proteins are not fixed in shape but flexible • Binding of the substrate at the active site causes it to bind more tightly Enzymes • Cofactors and coenzymes – Some enzymes need other molecules to function – Cofactors: inorganic molecules e.g. ions (Zn+, Ca+) – Coenzymes: organic molecules e.g. vitamins • identify the pH as a way of describing the acidity of a substance Factors affecting enzyme activity • Activity of enzymes are affected by: – temperature – pH – substrate concentration Temperature • Enzymes function best at the body temperature of the organism – Usually up to 40 °C • Most enzymes completely stop functioning above 60 °C because they become denatured – Proteins are held together by hydrogen bonds, these are disrupted at high temperatures pH • pH is a measure of the acidity or alkalinity of a substance – Acidity is caused by the presence of hydrogen ions (H+) • pH is the –log10[H+] – Therefore, the higher the concentration of H+, the lower the pH pH scale pH • As with temperature, enzymes function best within a narrow range of pH • Outside of the optimum pH, the enzyme becomes denatured • Most enzymes inside cells function around neutral pH • Enzymes in the stomach (pepsin) function best in acidic conditions whereas amylase, the enzyme in saliva that breaks down starch function in slightly alkaline conditions Substrate concentration • If an enzyme and substrate have a high affinity for each other, then the rate of an enzyme controlled reaction is affected by the concentration of the substrate until all enzymes are being used to catalyse reactions Substrate concentration • The saturation point is when all active sites are occupied by substrate and the reaction is proceeding at its maximum rate • In biological systems, the substrate is usually slightly in excess • identify data sources, plan, choose equipment or resources and perform a firsthand investigation to test the effect of: – increased temperature – change in pH – change in substrate concentrations on the activity of named enzyme(s) Enzyme: catalase • Catalase is found in potato or fresh plant or animal tissue • Converts hydrogen peroxide (H2O2) to water (H2O) and oxygen (O2) substrate concentration Results and discussion questions • Results: – Results table – Graph of concentration of H2O2 v. height of O2 gas • Discussion: – Questions from handout – What substrate concentration would you choose if you were investigating the effect of pH on catalase? Explain your choice. Enzyme: rennin • Rennin is found in junket tablets • It is used to set/curdle milk protein (caseinogen) temperature Results and discussion questions • Results: – Table of results – Graph of temperature v. activity (1/clotting time) • Discussion: – Questions from handout – If you place the tubes that didn’t clot into the waterbath with optimum activity, what would you expect to see? • Why would you do this? • What if the milk doesn’t clot at the optimum temperature? Enzyme: amylase • Amylase is found in saliva (and also commercially available) • Breaks down starch into simple sugars • Starch reacts with iodine (brown) to form a blue/black colour pH Sample data pH Time for complete breakdown of starch (s) 1 - 4 60 7 210 10 - 14 **iodine went colourless without enzyme • describe homeostasis as the process by which organisms maintain a relatively stable internal environment Homeostasis • Homeostasis = “same state” • It is “the maintenance by an organism of a constant or almost constant internal state, regardless of external environmental change” • Plants and animals have regulatory systems to maintain the internal balance of: – Temperature – Chemical substances (metabolites aka substrate) – Water and salt balance – (Absence of) waste and toxins Homeostasis • For example, in humans: – Blood sugar remains close to 90 mg/100 mL blood – Body temperature approximates 37°C – Blood pH must be within the range 7.38–7.42 regardless of external temperature, diet or physical activity. • explain why the maintenance of a constant internal environment is important for optimal metabolic efficiency Homeostasis and metabolic efficiency • Internal environment refers to: – Cells and their cytoplasm – Interstitial fluid surrounding the cells • All chemical reactions within cells must occur efficiently and be effectively co-ordinated to bring about optimal metabolic efficiency All chemical reactions necessary for the cell’s survival and functioning are controlled by enzymes Enzymes need a stable internal environment to function effectively E.g. Cellular respiration C6H12O6 + 6O2 6H2O + 6CO2 + energy (ATP) Importance of temperature and pH • Enzymes which need a narrow range of temperature and pH to function optimally • Outside of the optimal range, metabolic efficiency decreases • Low temperatures can freeze the water in cells which changes solute concentration and ruptures cells • High temperatures denature proteins (enzymes and others), disrupting cell function Importance of metabolites • Metabolites are the chemicals of a chemical reaction in the cell • Cells need the right amount of reactants which come from the outside environment or are products of other metabolic pathways – E.g. ATP is the energy produced from cellular respiration many reactions need ATP to proceed Importance of water and salt concentrations • Reactants are dissolved in water (in solution) so the amount of water affects the concentration of solutes • Salt concentrations affect the osmotic balance Osmotic balance • http://www.youtube.com/watch?v=t1nwSuW r_q8 Importance of absence of toxins • Build up of wastes (e.g. carbon dioxide) can be toxic to cells • They can interfere with enzymes by blocking the active site or change the condition of the environment (e.g. dissolved carbon dioxide decreases pH) • Therefore wastes must be removed to ensure optimal metabolic efficiency • explain that homeostasis consists of two stages: – detecting changes from the stable state – counteracting changes from the stable state Maintaining homeostasis • Enzyme activity depends on substrate concentration until saturation point is reached • Ideally the substrate concentration should be at a level where the enzyme is close to maximum capacity • Metabolic pathways use feedback mechanisms to regulate enzyme activity Homeostasis • In order to maintain homeostasis, an organism needs to: – Detect changes (stimulus) • Sensory cells or receptor cells detect changes in temperature or chemical composition – Counteract changes (response) • Effector organs (muscles or glands) work to reverse the change – The ideal or normal value is known as the set point • outline the role of the nervous system in detecting and responding to environmental changes Nervous system • A negative feedback system ensures homeostasis even when external conditions are changing – Coordinated by the nervous system: 1. Detects information 2. Transmits information to the control centre 3. Information is processed and response is generated Nervous system • Parts of the nervous system: – Receptors: sensory cells/organs – Control centre: central nervous system – Effectors: muscles/glands – Nerves: relay message • Stimulus-response pathway Thermoregulation in humans • What are some causes of temperature change in the body? 3 2 Thermoregulation in humans 1 1 3 2 • gather, process and analyse information from secondary sources and use available evidence to develop a model of a feedback mechanism Homeostasis analogy • Temperature of a refrigerator or waterbath • identify the broad range of temperatures over which life is found compared with the narrow limits for individual species Temperature ranges • Temperature is a limiting factor for the presence of living things • Temperatures on Earth: – Land: –89 to 60°C – Ocean: –2 to 30°C – Hydrothermal vents: >350°C Temperature ranges • Most living things live at temperatures between 10-35°C – Some organisms have evolved to live at extreme temperatures (extremophiles) Hydrothermal vents • http://www.deepseaphotography.com/vent_a nimals.html Narrow temperature limits for individual species • Optimal range – temperature range that a species can live comfortably • Tolerance range – range of temperatures for survival – The most temperature tolerant organism known is the Pompeii worm (22-80°C) • compare responses of named Australian ectothermic and endothermic organisms to changes in the ambient temperature and explain how these responses assist temperature regulation Ecto/endotherms • Ectothermic organisms depend on an external source for heat energy – E.g. Fish, amphibians, reptiles • Endotherms rely on internal sources such as metabolic activity for heat energy – E.g. Birds and mammals Ectothermic organisms • Internal temperature of these organisms are largely influenced by the ambient temperature • They have a limited ability to regulate their body temperature, instead they adapt their behaviour Eastern brown snake • Found in open grassland and desert scrub • Usually diurnal (awake during the day) – Can be active at night and seek shelter during day if day is too hot – If ambient temperature is too low, it will bask in sun to gain heat – When it is very cold, metabolism will slow down and use fat stores or snake will hibernate Endothermic organisms • They have the ability to control their body temperature and maintain it at a stable level • They adjust their metabolic rate to control heat loss – Small animals lose heat faster so they usually have a high metabolic rate Bentwing bat • Produce brown fat during the summer months when food is abundant • During colder months the brown fat can be quickly metabolised to produce heat and increase body temperature Fairy penguin • Their feathers trap heat around their body to reduce heat loss – In cold ambient temperatures, the feathers are lifted to increase the air layer and increase the insulation – In hot weather, their feathers lie flat • Behavioural mechanisms: – Swimming when hot – Huddling when cold • identify some responses of plants to temperature change Homeostasis in plants • Homeostasis in plants is also important for their metabolism – Light – Water availability – Temperature Responses to high temperatures • Evaporative cooling (transpiration) – Stomata open, leading to loss of water – BUT risk of dehydration Responses to high temperatures • Turgor response – wilting – Transpiration leads to water loss and leaves lose turgor – Wilted leaves have less surface area exposed to the sun Responses to high temperatures • Leaf orientation – Eucalypt leaves can hang vertically downwards in hot weather • Leaf fall – Eucalypt leaves can drop off to reduce exposure to the sun Responses to high temperatures • Reseeding and resprouting in fire – Bottle brush, tea trees and eucalypts, have epicormic buds or lignotubers which resprout after fire Responses to cold temperatures • Organic antifreeze – Reduces freezing temperature of cell cytoplasm or sap • Dormancy – Deciduous trees lose their leaves in winter and become dormant, sometimes seeds or spores are produced • Vernalisation – Some plants flower in response to low temperatures e.g. Tulips • analyse information from secondary sources to describe adaptations and responses that have occurred in Australian organisms to assist temperature regulation 2. Plants and animals transport dissolved nutrients and gases in a fluid medium • analyse information from secondary sources to identify the products extracted from donated blood and discuss the uses of these products • analyse and present information from secondary sources to report on progress in the production of artificial blood and use available evidence to propose reasons why such research is needed Blood products report • Weighting: 15% • Due: Week 8, Tuesday 25th November • Part A: Table of blood products • Part B: Development of artificial blood • identify the form(s) in which each of the following is carried in mammalian blood: – carbon dioxide – oxygen – water – salts – lipids – nitrogenous waste – other products of digestion Transport systems • Unicellular organisms rely on diffusion and osmosis • Multicellular organisms need a specialised transport system to distribute nutrients and remove wastes • Transport systems involve: 1. A transport medium 2. Vessels 3. A driving mechanism Mammalian transport • Cardiovascular system: – Heart – Blood vessels – Blood (medium of transport) Blood • Human adult has ~5L blood • pH of 7.35, 38°C • Blood consists of: – Blood cells (45%) – Plasma (55%) • Important role in homeostasis Blood composition • Blood cells – Red blood cells – White blood cells – Platelets – All blood cells are produced in the bone marrow Blood composition • Red blood cells – Aka erythrocytes – 4-6 million/mL blood – Transport oxygen – Initially have a nucleus but disappears as the cell matures – Contains haemoglobin, a red pigment – Lifespan of 4 months Blood composition • White blood cells – Aka leucocytes – 4000-11000/mL blood – Part of the immune system and help protect body from invading organisms Blood composition • Platelets – Aka thromobocytes – Disc shaped cell fragments, half size of RBCs – 400 000/mL blood – Help to clot blood by releasing the enzyme thromboplastin • perform a first-hand investigation using the light microscope and prepared slides to gather information to estimate the size of red and white blood cells and draw scaled diagrams of each Blood composition • Plasma – Yellow, watery fluid – 90% water, 10% proteins and solutes – Contains: • Proteins (clotting proteins, immunoglobulins, albumin, enzymes) • Nutrients • Gases • Excretory waste products • Ions • Regulatory substances (hormones) • Other (vitamins) Blood composition 1. Blood gases – Cellular respiration: • C6H12O6 + 6O2 6H2O + 6CO2 + energy (ATP) • http://ed.ted.com/lessons/what-do-the-lungsdo-emma-bryce • outline the need for oxygen in living cells and explain why removal of carbon dioxide from cells is essential Blood composition 1. Blood gases – Oxygen • Needed for respiration • Oxygen diffuses across the lung into the blood • 98.5% of the oxygen binds to haemoglobin (transport protein) oxyhaemoglobin (bright red) • 1.5% dissolved in plasma Blood composition 1. Blood gases – Carbon dioxide • Waste product of respiration • CO2 + H2O → H2CO3 → HCO3– + H+ • 70 % transported in blood as hydrogen carbonate ions (HCO3-) in plasma • 7% CO2 dissolved in plasma (carbonic acid, H2CO3) • 23% carried by haemoglobin carbaminohaemoglobin • perform a first-hand investigation to demonstrate the effect of dissolved carbon dioxide on the pH of water The effect of carbon dioxide on the pH of water • Results: a) b) c) Record the initial pH of the distilled water. Record the pH of the water after it contained dissolved carbon dioxide. State whether each is indicative of a strong or weak acidic or basic solution. • Discussion: 1. 2. 3. 4. Comment on the accuracy of the pH measurements that you have taken, and any difficulties that you had. Describe the effect of carbon dioxide on the pH of the water. Explain in chemical terms, what caused the change in the pH of the water in your experiment. Mammals try to maintain a blood pH of 7.4. Outline why they need to get rid of carbon dioxide from their cells as soon as possible Blood composition 2. Water and salts – Water is essential for transport of substances in: • • • Cytoplasm Interstitial fluid Blood – Salts are transported as ions • • E.g. NaCl is transported as Na+ and Cl- ions Aka electrolytes: Na+, K+, Cl-, HCO3- Blood composition 3. Lipids and other products of digestion – Digestion involves breaking down large molecules into smaller molecules: • Carbohydrates glucose • Lipids fatty acids and glycerol • Proteins amino acids • Nucleic acids nucleotides Blood composition 3. Lipids and other products of digestion – Soluble compounds (glucose, amino acids, glycerol and nucleotides) are dissolved in plasma and transported in the bloodstream – Lipids and end products are insoluble in water and need to be packaged for transport • “Chylomicrons” are carried by the lymphatic system Blood composition 4. Nitrogenous wastes – Produced from breakdown of proteins – Ammonia, urea, uric acid and creatinine – Dissolved in plasma (very dilute) and excreted from the body • explain the adaptive advantage of haemoglobin Haemoglobin (Hb) • Is a protein in RBCs which contains iron (Fe) • Adaptation of RBCs – No nucleus, more space for haemoglobin (250 million molecules/RBC) carry lots of oxygen – Biconcave shape of RBC increases surface area for oxygen diffusion Haemoglobin (Hb) • Each Hb binds to 4 oxygen molecules oxyhaemoglobin 1. Haemoglobin increases the oxygen carrying capacity of blood More oxygen can be transported than if dissolved in plasma 2. The ability to bind oxygen increases once the first oxygen molecule binds to it 3. Its capacity to release oxygen increases when carbon dioxide is present – Hb binds less strongly to O2 in acidic pH • compare the structure of arteries, capillaries and veins in relation to their function Transport systems 1. A transport medium (blood) 2. Vessels 3. A driving mechanism (heart) Cardiovascular system • Arteries • Veins • Capillaries How the heart pumps blood • http://ed.ted.com/lessons/how-the-heartactually-pumps-blood-edmond-hui Arteries • Function: transport blood away from the heart, towards the tissues • Structure: – Artery walls have 3 layers: 1. A thin inner layer of endothelial cells 2. A middle layer of smooth muscle and elastic fibres 3. An outer layer of connective tissue • Artery walls are thick due to the high pressure of the blood pumping through Veins • Function: transport blood towards the heart, away from the tissues • Structure: Same basic structure as arteries • Walls of veins are thinner than arteries because the blood travels under lower pressure • Fewer elastic fibres • Wider lumen Veins • Backflow is prevented in veins by: – Contraction of surrounding muscles – Valves in the endothelium lining Capillaries • Function: Tiny, thin-walled blood vessels in the tissues of the body, linking arteries and veins – Allow for exchange of chemical substances between cells and the bloodstream • Structure: – Capillary walls have an endothelium layer which is one cell thick – Allows one RBC through at a time • describe the main changes in the chemical composition of the blood as it moves around the body and identify tissues in which these changes occur Change in carbon dioxide and oxygen • Lungs: – External gas exchange – Deoxygenated blood arrives at the lungs, releases carbon dioxide and picks up oxygen – Most of the oxygen binds to haemoglobin to form oxyhaemoglobin. A small amount is dissolved in plasma. – Oxygenated blood is returned to the heart before it is pumped to the other tissues of the body Change in carbon dioxide and oxygen • Body tissues – Oxygenated blood arrives at the body tissues where oxygen is released and used for cellular respiration – Carbon dioxide is produced as a waste product of cellular respiration and is transported back to the lungs as bicarbonate ions, bound to haemoglobin or dissolved in plasma Other chemical changes • Products of digestion are increased in blood around the small intestines • Products of digestion decrease in blood which has passed through the liver (where food is metabolised) • Nitrogenous wastes increase in blood after it has passed through the liver • Nitrogenous wasted decrease in blood that has passed through the kidneys • analyse information from secondary sources to identify current technologies that allow measurement of oxygen saturation and carbon dioxide concentrations in blood and describe and explain the conditions under which these technologies are used • describe current theories about processes responsible for the movement of materials through plants in xylem and phloem tissue Transport in plants • Small plants use diffusion • Larger plants have specialised vascular tissue – Xylem and phloem – Movement of materials is called translocation • Needed for the translocation of substances for photosynthesis Xylem • Consists of: – Vessel structures tracheids and xylem vessels – Parenchyma cells – Fibres • Transport water, minerals and ions from roots to leaves for photosynthesis (unidirectional) Phloem • Consists of: – Sieve tube elements (alive) – Companion cells which help keep sieve tubes alive – Phloem fibres – Phloem parenchyma • Transport nutrients (sugars, amino acids, hormones) to all parts of the plant • Transport is bidirectional • choose equipment or resources to perform a first-hand investigation to gather first-hand data to draw transverse and longitudinal sections of phloem and xylem tissue Xylem and phloem in root cross section Xylem and phloem in stem cross section Xylem: transpiration stream theory (cohesion-adhesion-tension) 1. As the sun warms the leaves, stomata open and water evaporates through the openings (transpiration) 2. Evaporation at the leaf surface creates a pull at the upper end of the water column (tension) 3. The pulling force is extended to the water column and creates a force that pulls water upwards—the transpiration stream 4. This creates a force that pulls water into the roots Phloem: pressure flow theory (source-path-sink) • This is an active process which requires energy 1. Loading at the source – Amino acids, sucrose and other nutrients are loaded into the phloem in the leaves using ATP. – The solute concentration in the phloem increases and water moves into the phloem from the xylem by osmosis. Phloem: pressure flow theory (source-path-sink) 2. Offloading at the sink – Sugars and materials are removed from the phloem by active transport. – As a result, water is drawn out by osmosis. 3. Pressure flow along the ‘path’ – The movement of water creates an osmotic pressure gradient which drives the movement of phloem sap 3. Plants and animals regulate the concentration of gases, water and waste products of metabolism in cells and in interstitial fluid • explain why the concentration of water in cells should be maintained within a narrow range for optimal function Excretion • The process by which waste products are removed from the body • Waste products need to be removed because they affect the internal environment of the cell • Organs of the excretory systems in mammals include: – Lungs (CO2) – Kidneys (nitrogenous wastes) – Skin (salt) Why is water important? • Water makes up 2/3 of the composition of most living organisms • Water is a solvent • Makes up the cytoplasm and body fluids – Carries ions, organic solutes and wastes • Transport medium in plants – Carries ions and sugars Why is water important? • Changes in concentration of water: – Changes the osmotic balance • Leads to a change in solute concentration • Or cells can burst • Needed for structural support Why is water important? • All chemical reactions of metabolism take place in water • Reactants and products are dissolved in water – E.g. CO2 affect pH • Water sometimes take place in reactions e.g. photosynthesis • explain why the removal of wastes is essential for continued metabolic activity Accumulation of wastes • Wastes may be toxic to cells and must be removed from the body to maintain homeostasis. • If their levels increase, the internal environment will change ?? • distinguish between active and passive transport and relate these to processes occurring in the mammalian kidney Kidneys • Organs responsible for removing nitrogenous waste from the body by filtering blood • The renal artery carries oxygenated blood to the kidneys • The kidneys filter out nitrogenous wastes, water and other solutes • Urine drains via the ureters to the bladder • The renal vein carries purified blood back into circulation active vs passive transport? diffusion vs osmosis? Passive transport in the kidney • Nitrogenous wastes (ammonia and urea) and water move by diffusion and osmosis from the blood to the kidney tubules • Salts (active) and water (passive) can be reabsorbed depending on the need to maintain homeostasis. Active transport in the kidney • Active transport requires energy and involves the movement from low concentration to high concentration • Involves carrier proteins which actively move: – All glucose and amino acids back into the blood – Ions (salts) back into the blood – Additional nitrogenous wastes and hydrogen ions into the urine • explain why the processes of diffusion and osmosis are inadequate in removing dissolved nitrogenous wastes in some organisms Diffusion and osmosis: inadequate • Diffusion and osmosis are passive so they are slow • Diffusion relies on concentration gradient but net movement stops when equilibrium is reached not all wastes will be removed even small amounts of wastes can be toxic • Diffusion of wastes into urine will also draw water into the urine by osmosis lots of water loss • gather, process and analyse information from secondary sources to compare the process of renal dialysis with the function of the kidney • perform a first-hand investigation of the structure of a mammalian kidney by dissection, use of a model or visual resource and identify the regions involved in the excretion of waste products • explain how the processes of filtration and reabsorption in the mammalian nephron regulate body fluid composition Nephron structure Function of nephron • Carries out 3 processes: – Filtration – Reabsorption – Secretion 1. Filtration • Renal artery branches out into many capillaries which end at the glomerulus • Blood is filtered by high pressure across the surface of the glomerulus and the inner lining of the Bowman’s capsule, removing: – – – – – – Water Amino acids Glucose Salts Nitrogenous wastes Other toxic molecules glomerular filtrate 2. Reabsorption • Regulates chemical composition of body fluids • Active transport • Membrane permeability is regulated by hormones to control what is reabsorbed • 99% of filtrate is reabsorbed • Reabsorption of water is passive as it follows the movement of the solutes 2. Reabsorption • Reabsorption of certain molecules occur at different parts of the nephron: – All organic nutrients (amino acids and glucose) Proximal tubule – Some ions (Na+, K+, Cl-, Ca2+, HCO3-) Proximal tubule – Water Descending limb of loop of Henle All parts of tubule except the ascending limb of loop of Henle – Na+ Collecting tubules 3. Secretion • Active secretion of toxic substances into the nephron: – Metabolic wastes (urea, uric acid, ammonia, H+) • H+ proximal tubule • Urea Descending limb of loop of Henle – Drugs (penicillin, saccharine, morphine) Proximal tubule • outline the role of the hormones, aldosterone and ADH (anti-diuretic hormone) in the regulation of water and salt levels in blood Stop drinking water • http://www.youtube.com/watch?v=zCheAcpF kL8 Hormone regulation of excretion • Hormones are secreted by endocrine glands and travel through the blood stream • Adjustments to water and salt in the urine are controlled at the distal tubules and collecting tubules by changing the permeability of the membrane • Aldosterone: conserves salts in the body • ADH (anti-diuretic hormone): conserves water in the body Aldosterone • ↓[Na+] (sodium ion concentration) ↓blood volume Stimulates adrenal gland (above kidney) Aldosterone is secreted At the kidney, the nephron becomes more permeable to sodium, especially at the ascending limb ↑reabsorption of sodium ions Less salt is lost in urine, and salt is retained by the body ADH • Dehydration ↓blood volume Detected by hypothalamus Stimulates pituitary gland ADH is released At the kidney, the nephron becomes more permeable to water at the distal tubules and collecting tubules ↑ reabsorption of water Water is retained in the body • present information to outline the general use of hormone replacement therapy in people who cannot secrete aldosterone • identify the role of the kidney in the excretory system of fish and mammals Role of kidney in fish and mammals • Excretory and osmoregulatory organs • Mammals: – Conserve water and salts (when required) – Excrete excess water and salts – Excrete nitrogenous wastes Role of kidney in fish and mammals • Freshwater fish: – Excrete excess water – Excrete nitrogenous wastes – Conserve salt • Excess salt is excreted via gills (when needed) Role of kidney in fish and mammals • Marine fish: – Conserve water – Excrete excess salt via gills – Excrete nitrogenous wastes • analyse information from secondary sources to compare and explain the differences in urine concentration of terrestrial mammals, marine fish and freshwater fish • use available evidence to explain the relationship between the conservation of water and the production and excretion of concentrated nitrogenous wastes in a range of Australian insects and terrestrial mammals • define enantiostasis as the maintenance of metabolic and physiological functions in response to variations in the environment and discuss its importance to estuarine organisms in maintaining appropriate salt concentrations Enantiostasis – estuarine organisms • Tides change the salinity of estuaries from day to day – High tide high salt in water – Low tide low salt in water Enantiostasis – estuarine organisms • Osmoregulation is maintained by 2 different strategies: 1. Osmoconformers: • Alter internal concentrations of solutes to match external environment 2. Osmoregulators: • Change internal environment to maintain solutes at an optimal level Enantiostasis – estuarine organisms Osmoconformers • Fiddler crab – Accumulates solutes in tissues in saltwater – Excretes salt through gills in freshwater Osmoregulators • Mussels – Close their valves in freshwater to keep internal conditions same as seawater • describe adaptations of a range of terrestrial Australian plants that assist in minimising water loss Balance in Australian plants • Australian plants need to maintain a balance between: – Water loss by transpiration (98%) and evaporative cooling to maintain optimal temperature – Surface area exposed to the sun and surface area needed for photosynthesis – Closing stomata to conserve water and opening stomata for gas exchange • Xerophytes are a species of plant adapted to hot, dry conditions Adaptations 1. Reducing internal temperature – Waxy leaves reflect light and heat • E.g. saltbush – Coarse, leathery leaves with thick cuticle provides insulation from sunlight • E.g. sclerophylls such as eucalypts and banksias Adaptations 2. Reducing exposure to sunlight – Reduce surface area of organs that have a lot of stomata (leaves) • • E.g. Wattles have reduced leaves Leaves are so reduced in some plants that photosynthesis is carried out by the stem (cladodes) or leaf stalks (phyllodes) – Change orientation of leaves • E.g. Eucalypts Adaptations 2. Reducing exposure to sunlight – Absence of transpiring organs • • Reduced flowers or no petals E.g. Wattles (acacias) – Shedding leaves • E.g. River gum Adaptations 3. Reducing the water potential – Water potential is the difference in water concentration between the plant and the environment – Plants create microclimates, trapping moisture around the leaves to reduce the water potential: • Sunken stomata: E.g. Hakea Adaptations 3. Reducing the water potential • Epidermal hairs: E.g. Coastal banksia • Curled or rolled leaves: E.g. porcupine grass Adaptations 4. Water storage – Plants such as succulents have fleshy leaves or stems that can hold water which can be used during dry periods • E.g. Calandrinia (parakeelya) • process and analyse information from secondary sources and use available evidence to discuss processes used by different plants for salt regulation in saline environments • perform a first-hand investigation to gather information about structures in plants that assist in the conservation of water