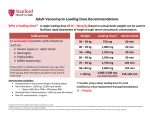
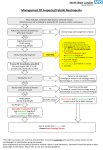
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Vancomycin: A 50-Year Reassessment
Survey
Document related concepts
Transcript
INTRODUCTION SUPPLEMENT ARTICLE Vancomycin: A 50-Year Reassessment Robert C. Moellering, Jr. Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts More than half a century ago, the compound now known as vancomycin was isolated from a soil sample collected deep in the interior jungle of Borneo. The isolation was performed by Dr. E. C. Kornfeld, an organic chemist at Eli Lilly, which had begun a major program to discover new antimicrobial agents with activity against staphylococci [1]. Although it had been only 15 years since the initial deployment of penicillin and the subsequent discovery of macrolides and tetracyclines, staphylococcal resistance to these compounds was already a major problem in hospitals throughout the world. The soil sample from Borneo contained an organism (subsequently named “Streptomyces orientalis”) that yielded a compound in broth fermentation with a high degree of bactericidal activity against staphylococci. The initial compound was labeled 05865, and early laboratory studies showed that staphylococci failed to develop significant resistance to 05865 on serial passage in culture media containing the drug. Because of the growing menace of drug-resistant staphylococci, the US Food and Drug Administration essentially “fast-tracked” approval of compound 05865, which was subsequently given the generic name “vancomycin,” a term derived from the word “vanquish.” The orig- Reprints or correspondence: Dr. Robert C. Moellering, Jr., Dept. of Medicine, Beth Israel Deaconess Medical Center, 110 Francis St., Ste. 6A, Boston, MA 02215 (rmoeller@ bidmc.harvard.edu). Clinical Infectious Diseases 2006; 42:S3–4 2005 by the Infectious Diseases Society of America. All rights reserved. 1058-4838/2006/4201S1-0002$15.00 inal preparations of vancomycin from fermentation broth contained a number of impurities, and, because of the brown color of the material, it was nicknamed “Mississippi mud” by scientists at Eli Lilly [1]. Despite its early promise, however, vancomycin was not widely used in the decade following its discovery. The major reasonwas that methicillin and, subsequently, other antistaphylococcal penicillins were discovered and became the drugs of choice for treating staphylococcal infections. Vancomycin was relegated to a secondary role, in large part, on the basis of results of early studies performed during the mid-1950s that showed it to be ototoxic and nephrotoxic [1]. It is very likely that whatever ototoxicity and nephrotoxicity resulted from the use of vancomycin were related to the presence of impurities in the earlier preparations; when newer, purer preparations were retested in the late 1970s, they produced no ototoxicity and little nephrotoxicity in the animal models, unless given in combination with aminoglycosides [2, 3]. Because of the possible toxicity of vancomycin, it was not heavily marketed during the 1960s and 1970s and was relegated to a secondary role in antibacterial chemotherapy. However, the worldwide emergence of methicillin-resistant staphylococci in the 1970s rekindled interest in vancomycin. It was only at this juncture that the pharmacokinetics of the drug were determined, and the first of several nomograms for dosage for patients with impaired renal function was published [4]. It was also at this point that the initial reevaluation of vancomycin was published as a supple- ment in Reviews of Infectious Diseases (the predecessor to Clinical Infectious Diseases). The supplement was entitled “Reassessments of Vancomycin—A Potentially Useful Antibiotic” [5] and reflected a cautious, somewhat measured approach to the potential utility of vancomycin. That publication also marked the first quarter century of the existence of vancomycin as an antimicrobial agent—a period during which it was little more than a “slumbering non-giant.” Things changed rapidly in the 1980s, however, and the worldwide use of vancomycin accelerated. Another glycopeptide, teicoplanin, was also developed and used widely during that time in most countries, except the United States, where it was never licensed. Because vancomycin was no longer patented by that time, it was not subjected to the intense marketing typically associated with antimicrobial agents. Nonetheless, the drug more than “sold itself” because of a very real clinical need. As predicted from earlier in vitro studies, there was initially little emergence of resistance to vancomycin or teicoplanin among staphylococci and other gram-positive bacteria. However, the emergence of vancomycin-resistant enterococci in the middle of the 1980s served as a wake-up call [6]. It took more than a decade until significant resistance to vancomycin was also discovered in staphylococci. These resistant isolates were initially termed “vancomycin-intermediate Staphylococcus aureus,” because the MICs of vancomycin for these organisms were in the “intermediate” category of the arbitrarily assigned Introduction: Vancomycin • CID 2006:42 (Suppl 1) • S3 vancomycin susceptibility breakpoints [7]. Nonetheless, these organisms were clinically resistant to vancomycin, and patients infected with these organisms often experienced failure of therapy with vancomycin [8]. True high-level resistance to vancomycin in S. aureus, which is due to the acquisition of vanA genes, presumably from enterococci, first occurred in 2002 in Michigan [9]. Although this event served as a wake-up call that high-level vancomycin resistance in S. aureus was possible, to date, this has not become a significant clinical problem, because only 5 isolates (all from the United States) have been documented. Nonetheless, more subtle hints of clinical failure of vancomycin have begun to emerge, and there is growing evidence that vancomycin may not be as effective against all strains of staphylococci as it was 25 years ago [10]. At this point, the true significance of this apparently diminished clinical activity of vancomycin (and teicoplanin) remains to be firmly defined, but it is of concern. Thus, 50 years after its discovery, van- S4 • CID 2006:42 (Suppl 1) • Moellering, Jr. comycin remains an interesting and even somewhat controversial agent. However, we know a great deal more about the drug than we did 25 years ago. It seems more than reasonable at the half-century mark to once again reassess the effectiveness and utility of vancomycin. The articles in this supplement deal with a number of important issues relating to the development, pharmacodynamics, safety, and therapeutic efficacy of modern preparations of vancomycin and should serve to provide an effective framework for determining its appropriate clinical niche in the coming years. 4. 5. 6. 7. 8. Acknowledgment Potential conflicts of interest. R.C.M., Jr. has served as a consultant to Pfizer, Cubist, and Vircuron. References 1. Griffith RS. Introduction to vancomycin. Rev Infect Dis 1981; 3:S200–4. 2. Brummett RE. Effects of antibiotic-diureticinteractions in the guinea pig model of ototoxicity. Rev Infect Dis 1981; 3:S216–23. 3. Farber BF, Moellering RC Jr. Retrospective 9. 10. study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983; 23:138–41. Moellering RC Jr, Krogstad DJ, Greenblatt DJ. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med 1981; 94:343–6. Reassessments of vancomycin—a potentially useful antibiotic. Rev Infect Dis 1981; 3: S190–300. Leclerq R, Derlot E, Duval J, et al. Plasmidmediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med 1988; 319:157–61. Hiramatsu K, Hanaki H, Ino T, et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 1997; 40:135–6. Fridkin SK, Hageman J, McDougal LK, et al. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis 2003; 36:429–39. Centers for Disease Control and Prevention (CDC). Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep 2002; 51:565–7. Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM. Relationship of minimal inhibitory concentration (MIC) and bactericidal activity to efficacy of vancomycin for treatment of methicillinresistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–402.