* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IUPAC Provisional Recommendations
Survey
Document related concepts
Transcript
1
Preface
Nomenclature
of
Inorganic
Chemistry
-
s
Since the publication of IUPAC's
tio
n
Recommendations 1990 (the 'Red Book') inorganic chemistry has continued to expand and
flourish, bringing with it the need to adapt and develop associated nomenclature. A revision
of the Red Book was therefore initiated in 1998, under the guidance of the IUPAC
da
Commission on Nomenclature of Inorganic Chemistry (CNIC) and then, on the abolition of
om
me
n
CNIC in 2001, by a project group working under the auspices of the Division of Chemical
Nomenclature and Structure Representation (Division VIII).
The need to ensure that inorganic and organic nomenclature systems are, as far as
possible, consistent has resulted in extensive cooperation between the editors of the new
Red Book and the revised Nomenclature of Organic Compounds (the 'Blue Book'). At
present, the concept of preferred IUPAC names (PINs), an important element of the current
ec
revision of the Blue Book, has not been extended to inorganic nomenclature (though
lR
preferred names are used herein for organic, i.e. carbon-containing, compounds when
appropriate). A future project on inorganic PINs is planned but will need to face the
na
problem of choice between the equally valid nomenclature systems currently in use.
The current book supersedes not only the 1990 Red Book but also, where
isi
o
appropriate, Nomenclature of Inorganic Chemistry II - Recommendations 2000 (Red Book
II). One of the main changes from the old Red Book is the different organization of
ov
material, adopted to improve clarity. Thus, Chapters IR-5 (Compositional Nomenclature and
Overview of Names of Ions and Radicals), IR-6 (Parent Hydride Names and Substitutive
Pr
Nomenclature), and IR-7 (Additive Nomenclature) deal with the general characteristics of
the three main nomenclature systems applied to inorganic compounds. (Note the notation
PA
C
'IR-' to distinguish sections in the current book from those in the previous version, prefixed
'I-'). The next three chapters deal with their application, particularly additive nomenclature, to
three large classes of compounds: inorganic acids (Chapter IR-8), coordination compounds
IU
(Chapter IR-9) and organometallic compounds (Chapter IR-10). Overall, the emphasis on
Page 1 of 4
DRAFT 2 April 2004
2
additive nomenclature (generalized from the classical nomenclature of coordination
s
compounds) which was already apparent in the 1990 Red Book, is reinforced here.
tio
n
Examples are even included of organic compounds, from the borderline between inorganic
and organic chemistry, which may be conveniently named using additive nomenclature
(although their PINs will be different).
da
One important addition in this book is Chapter IR-10 on Organometallic
om
me
n
Compounds, its separation from Coordination Compounds (Chapter IR-9) reflecting the
huge growth in importance of organometallic chemistry and the very different problems
associated with the presence of π-bonded ligands. Chapter IR-9 has also undergone
considerable revision (cf. the old Chapter I-10), changes including the alphabetical ordering
of ligands in names (and in formulae – see below) to avoid the ambiguity associated with
ec
assigning charge (Section IR-9.2.2.1), a clarification of the use of η and κ nomenclature in
coordination and organometallic compounds (Section IR-9.2.4.3), the ordering of central
lR
atoms in polynuclear compounds (Section IR-9.2.5.1), the bringing together of sections on
configuration (Section IR-9.3) and their separation from those on constitution (Section IR-
na
9.3), and the addition of polyhedral symbols for T-shaped (Section IR-9.3.3.7) and see-saw
(Section IR-9.3.3.8) molecules with guidance on how to chose between these and closely
isi
o
related structures (Section IR-9.3.2.2).
The chapter on Oxoacids and Derived Anions (I-9) has also been extensively
ov
modified. Now called Inorganic Acids and Derivatives (Chapter IR-8), it includes the
slightly revised concept of 'Hydrogen Names' in Section IR-8.4 (and some traditional 'ous'
Pr
and 'ic' names have been reinstated as they are required for organic nomenclature purposes,
i.e. in the new Blue Book).
PA
C
The reader facing the problem of how to name a given compound or species may
find help in several ways. A flowchart is provided in Section IR-I.4 which will in most cases
guide the user to a section or chapter where rules can be found for generating at least one
IU
possible name. A more detailed subject index is also provided in this book, and an extended
DRAFT 2 April 2004
Page 2 of 4
3
guide to possible alternative names of a wide range of simple inorganic compounds may be
s
found in Table IX.
tio
n
For most compounds, formulae are another important type of compositional or
structural representation and for some compounds a formula is perhaps easier to construct.
In Chapter IR-4 (Formulae) several changes are made in order to make the presentation of a
da
formula and its corresponding name more consistent, e.g. the order of ligand citation (which
om
me
n
does not now depend on the charge on the ligand) (Section IR-4.4.3.2) and the order and
use of enclosing marks (simplified and more consistent with the usage proposed for the
nomenclature of organic compounds) (Section IR-4.2.3). In addition, the use of ligand
abbreviations can make formulae less cumbersome. Thus, recommendations for the
construction and use of abbreviations are provided in Section IR-4.4.4, with an extensive list
of established abbreviations given in Table VII (and with structural formulae for the ligands
ec
given in Table VIII).
lR
Two chapters of the 1990 Red Book have been shortened or subsumed since in both
areas extensive revision is still necessary. First, the chapter on Solids (IR-11) now describes
na
only basic topics, more recent developments in this area tending to be covered by
publications from the International Union of Crystallography (IUCr). It is to be hoped that
isi
o
future cooperation between IUPAC and IUCr will lead to the additional nomenclature
required for the rapidly expanding field of solid state chemistry,
ov
Second, boron chemistry, particular of polynuclear compounds, has also seen
extensive development. Again, therefore, only the basics of the nomenclature of boron-
Pr
containing compounds (cf. the separate chapter on boron nomenclature, I-11, in the old Red
Book) are covered here, within Chapter IR-6 (Parent Hydride Names and Substitutive
PA
C
Nomenclature) while more advanced aspects are left for elaboration in a future project.
Other changes include sections on new elements and the procedure by which they
are now named (Section IR-3.1) and a simplified coverage of the systematic method for
IU
naming rings and chains (adapted from Chapter II-5 of Red Book II). Lesser omissions
Page 3 of 4
DRAFT 2 April 2004
4
include the section on single strand polymers (now updated as Chapter II-7 in Red Book II)
s
and the several different outdated versions of the Periodic Table. (That in the front inside
tio
n
cover is the IUPAC-agreed version). Some new recommendations represent breaks with
tradition, in the interest of increased clarity and consistency. For example, the application of
the ending 'ido' to all anionic ligands with 'ide' names in additive nomenclature (e.g. chlorido
da
and cyanido instead of chloro and cyano, and hydrido throughout, i.e. no exception in boron
om
me
n
nomenclature) is a move to a more systematic approach.
Thanks
We would like to thank the many people without whose help this revision would not
have come to fruition. Members of CNIC were involved in the early stages of this work
(including Stanley Kirschner who began the task of compiling ligand abbreviations and
ec
what has become Tables VII and VIII), and members of the IUPAC Division VIII Advisory
lR
Subcommittee (particularly Jonathan Brecher, Piroska Fodor-Csanyi and Alan McNaught)
and the authors of the revised Blue Book (Warren Powell and Henri Favre) have made
na
extremely valuable comments. However, the bulk of the work has been carried out by a
Neil G. Connelly and Ture Damhus
IU
PA
C
Pr
ov
isi
o
project group comprising the editors, Richard Hartshorn, Alan Hutton and Risto Laitinen.
DRAFT 2 April 2004
Page 4 of 4
IUPAC Periodic Table of the Elements
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
o
i
at
1
2
H
He
3
4
Li
Be
11
12
Na
Mg
19
20
21
22
23
24
25
26
27
28
29
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
37
38
39
40
41
42
43
44
45
46
47
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
55
56
* 57
72
73
74
75
76
77
Cs
Ba
La
Hf
Ta
W
Re
87
88
‡ 89
104
105
Fr
Ra
Ac
Rf
Db
I
Page 1 of 1
C
A
P
U
107
Sg
Bh
6
7
8
9
B
C
N
O
F
13
14
15
16
17
18
Al
Si
P
S
Cl
Ar
31
32
33
34
35
36
Ga
Ge
As
Se
Br
Kr
48
49
50
51
52
53
54
Ag
Cd
In
Sn
Sb
Te
I
Xe
78
79
80
81
82
83
84
85
86
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
108
109
110
111
112
113
114
115
116
117
118
Hs
Mt
Ds
Uuu
Uub
Uut
Uuq
Uup
Uuh
Uus
Uuo
Os
l
a
n
io
s
i
v
o
r
P
106
d
n
e
m
m
o
c
5
30
e
R
Zn
* 57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
‡ 89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
Ac
DRAFT 2 April 2004
s
n
n
1
10
Ne
2
3
4
5
6
7
6
7
1
General Aims, Functions and Methods of Chemical
Nomenclature (March 2004)
s
IR-1
tio
n
CONTENTS
INTRODUCTION
isi
o
IR-1.1
na
lR
ec
om
me
n
da
IR-1.1 Introduction
IR-1.2 History of chemical nomenclature
IR-1.2.1 International cooperation on inorganic nomenclature
IR-1.3 Aims of chemical nomenclature
IR-1.4 Functions of chemical nomenclature
IR-1.5 Methods of inorganic nomenclature
IR-1.5.1 Formulation of rules
IR-1.5.2 Name construction
IR-1.5.3 Systems of nomenclature
IR-1.5.3.1 General
IR-1.5.3.2 Compositional nomenclature
IR-1.5.3.3 Substitutive nomenclature
IR-1.5.3.4 Additive nomenclature
IR-1.5.3.5 General naming procedures
IR-1.6 Nomenclature recommendations in other areas of chemistry
IR-1.7 References
IU
IR-1.2
PA
C
Pr
ov
This Chapter provides a brief historical overview of chemical nomenclature followed by a
summary of its aims, functions and methods. There are several systems of nomenclature
that can be applied to inorganic compounds, briefly described here as an introduction to
the later, more detailed, chapters. Because each system can provide a valid name for a
compound, a flowchart is presented which should help identify which is the most
appropriate for the type of compound of interest. Finally, reference is made to
nomenclature recommendations in other areas of chemistry, underlining that inorganic
chemistry is part of an integrated whole.
Page 1 of 12
HISTORY OF CHEMICAL NOMENCLATURE
The activities of alchemy and of the technical arts practised prior to the founding of what
we now know as the science of chemistry produced a rich vocabulary for describing
chemical substances although the names for individual species gave little indication of
composition. However, almost as soon as the true science of chemistry was established a
'system' of chemical nomenclature was developed by Guyton de Morveau in 1782.1
Guyton's statement of the need for a 'constant method of denomination, which helps the
DRAFT 2 April 2004
2
om
me
n
da
tio
n
s
intelligence and relieves the memory' clearly defines the basic aims of chemical
nomenclature. His system was extended by a joint contribution2 with Lavoisier, Berthollet,
and de Fourcroy and was popularized by Lavoisier.3 Later, Berzelius championed
Lavoisier's ideas, adapting the nomenclature to the Germanic languages,4 expanding the
system and adding many new terms. This system, formulated before the enunciation of the
atomic theory by Dalton, was based upon the concept of elements forming compounds
with oxygen, the oxides in turn reacting with each other to form salts; the two-word names
in some ways resembled the binary system introduced by Linnaeus (Carl von Linné) for
plant and animal species.
ec
When atomic theory developed to the point where it was possible to write specific
formulae for the various oxides and other binary compounds, names reflecting
composition more or less accurately then became common; no names reflecting the
composition of the oxosalts were ever adopted, however. As the number of inorganic
compounds rapidly grew, the essential pattern of nomenclature was little altered until near
the end of the 19th century. As a need arose, a name was proposed and nomenclature grew
by accretion rather than by systematization.
na
lR
When Arrhenius focused attention on ions as well as molecules, it became necessary to
name charged particles in addition to neutral species. It was not deemed necessary to
develop a new nomenclature for salts; cations were designated by the names of the
appropriate metal and anions by a modified name of the non-metal portion.
International cooperation on inorganic nomenclature
IU
PA
C
IR-1.2.1
Pr
ov
isi
o
Along with the theory of coordination, Werner proposed5 a system of nomenclature for
coordination compounds which not only reproduced their compositions but also indicated
many of their structures. Werner's system was completely additive in that the names of the
ligands were cited, followed by the name of the central atom modified by the suffix 'ate' if
the complex was an anion. Werner also used structural descriptors and locants. The
additive nomenclature system was capable of expansion and adaptation to new compounds
and even to other fields of chemistry.
In 1892 a conference in Geneva6 laid the basis for an internationally accepted system of
organic nomenclature, but at that time there was nothing comparable for inorganic
nomenclature. Thus, many ad hoc systems had developed for particular rather than general
purposes, and two or more methods often evolved for naming a given compound
belonging to a given class. Each name might have value in a specific situation, or be
preferred by some users, but there was then the possibility of confusion.
DRAFT 2 April 2004
Page 2 of 12
3
da
tio
n
s
The need for a uniform practice among English-speaking chemists was recognized as early
as 1886 and resulted in agreements on usage by the British and American Chemical
Societies. In 1913, the Council of the International Association of Chemical Societies
appointed a commission of inorganic and organic nomenclature, but World War I abruptly
ended its activities. Work was resumed in 1921 when IUPAC, at its second conference,
appointed commissions on the nomenclature of inorganic, organic, and biological
chemistry.
om
me
n
The first comprehensive report of the inorganic commission, in 1940,7 had a major effect
on the systematization of inorganic nomenclature and made many chemists aware of the
necessity for developing a more fully systematic nomenclature. These IUPAC
recommendations were then revised and issued as a small book in 19598 followed by a
second revision in 19719 and a supplement, entitled How to Name an Inorganic Substance,
in 1977.10 In 1990 the IUPAC recommendations were again fully revised11 in order to
bring together the many and varied changes which had occurred in the previous 20 years.
isi
o
na
lR
ec
Since 1990, more specialized areas have been considered, concerning polyanions,12 metal
complexes of tetrapyrroles (based on Ref. 13), inorganic chain and ring compounds,14 and
graphite intercalation compounds.15 These topics, together with revised versions of papers
on isotopically modified inorganic compounds,16 hydrides of nitrogen and derived cations,
anions and ligands,17 and regular single-strand and quasi single-strand inorganic and
coordination polymers,18 comprise the seven chapters of Nomenclature of Inorganic
Chemistry II, Recommendations 2000.19 A paper entitled Nomenclature of Organometallic
Compounds of the Transition Elements20 forms the basis for Chapter IR-10.
IR-1.3
AIMS OF CHEMICAL NOMENCLATURE
IU
PA
C
Pr
ov
The primary aim of chemical nomenclature is simply to provide methodology for
assigning descriptors (names and formulae) to chemical substances so that they can be
identified without ambiguity, thereby facilitating communication. A subsidiary aim is to
achieve standardization. Although this need not be so absolute as to require only one name
for a substance, the number of 'acceptable' names needs to be minimized.
Page 3 of 12
When developing a system of nomenclature, public needs and common usage must also be
borne in mind. In some cases, the only requirement may be to identify a substance,
essentially the requirement prior to the late 18th century. Thus, local names and
abbreviations are still used by small groups of specialists. Such local names suffice as long
as the specialists understand the devices used for identification. However, this is not
nomenclature as defined above since local names do not necessarily convey structural and
compositional information to a wider audience. To be widely useful, a nomenclature
DRAFT 2 April 2004
4
FUNCTIONS OF CHEMICAL NOMENCLATURE
tio
n
IR-1.4
s
system must be recognisable, unambiguous, and general; the unnecessary use of local
names and abbreviations in formal scientific language should therefore be discouraged.
om
me
n
da
The first level of nomenclature, beyond the assignment of totally trivial names, gives some
systematic information about a substance but does not allow the inference of composition.
Most of the common names of the oxoacids (e.g. sulfuric acid, perchloric acid) and of their
salts are of this type. Such names may be termed semi-systematic and as long as they are
for commonly used materials and understood by chemists, they are acceptable. However,
it should be recognised that they may hinder compositional understanding by those with
limited chemical training.
When a name itself allows the inference of the stoichiometric formula of a compound
according to general rules, it becomes truly systematic. Only a name at this second level of
nomenclature becomes suitable for retrieval purposes.
na
lR
ec
The desire to incorporate information concerning the three-dimensional structures of
substances has grown rapidly and the systematization of nomenclature has therefore had to
expand to a third level of sophistication. Few chemists want to use such a degree of
sophistication every time they refer to a compound, but they may wish to do so when
appropriate.
isi
o
A fourth level of nomenclature may be required for the compilation and use of extensive
indexes. Because the cost to both compiler and searcher of multiple entries for a given
substance may be prohibitive, it becomes necessary to develop systematic hierarchical
rules that yield a unique name for a given substance.
METHODS OF INORGANIC NOMENCLATURE
IR-1.5.1
Formulation of rules
Pr
ov
IR-1.5
IU
PA
C
The revision of nomenclature is a continuous process as new discoveries make fresh
demands on nomenclature systems. IUPAC, through the Division of Chemical
Nomenclature and Structure Representation (formed in 2001), studies all aspects of the
nomenclature of inorganic and other substances, recommending the most desirable
practices to meet specific problems, for example for writing formulae and generating
names. New nomenclature rules need to be formulated precisely, to provide a systematic
basis for assigning names and formulae within the defined areas of application. As far as
possible, however, they should be consistent with existing recommended nomenclature, in
both inorganic and other areas of chemistry, and take into account emerging chemistry.
DRAFT 2 April 2004
Page 4 of 12
5
Name construction
da
IR-1.5.2
tio
n
s
Recommendations may incorporate common usage when it is systematic and
unambiguous; nomenclature developed in isolation from experimental chemistry will be
perceived as an imposition and therefore irrelevant to science. At the same time, the
development of new rules may also require a more rigorous definition of existing rules to
avoid inconsistencies, ambiguities, and the proliferation of names.
om
me
n
The systematic naming of an inorganic substance involves the construction of a name from
units which are manipulated in accordance with defined procedures to provide
compositional and structural information. The element names (or roots derived from them
or from their Latin equivalents) (Tables I and II*, see also Chapter IR-3) are combined
with affixes in order to construct systematic names by procedures which are called
systems of nomenclature.
ec
There are several accepted systems for the construction of names, as discussed in Section
IR-1.5.3. Perhaps the simplest is that used for naming binary substances. This set of rules
leads to a name such as iron dichloride for the substance FeCl2; this name involves the
na
lR
juxtaposition of element names (iron, chlorine), their ordering in a specific way
(electropositive before electronegative), the modification of an element name to indicate
charge (the 'ide' ending designates an elementary anion and, more generally, an element
being treated formally as anion), and the use of the numerical prefix 'di' to indicate
composition.
Pr
ov
isi
o
Whatever the pattern of nomenclature, names are constructed from units which fall into
the following classes:
element name roots,
numerical prefixes,
locants,
prefixes indicating atoms or groups _ either substituents or ligands,
IU
PA
C
suffixes indicating charge,
suffixes indicating characteristic substituent groups,
infixes,
additive prefixes,
subtractive suffixes/prefixes,
descriptors (structural, geometric, stereochemical, etc.),
punctuation.
The uses of these units are summarized in Chapter IR-2.
*
Page 5 of 12
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
6
Systems of nomenclature
IR-1.5.3.1
General
s
IR-1.5.3
om
me
n
da
tio
n
In the development of nomenclature, several systems have emerged for the construction of
chemical names; each system has its own inherent logic and set of rules (grammar). Some
systems are broadly applicable whereas practice has led to the use of other specialized
systems in particular areas of chemistry. The existence of several distinct nomenclature
systems leads to logically consistent alternative names for a given substance. Although
this flexibility is useful in some contexts, the excessive proliferation of alternatives can
hamper communication and even impede trade and legislation procedures. Confusion can
also occur when the grammar of one nomenclature system is mistakenly used in another,
leading to names that do not represent any given system.
Three systems are of primary importance in inorganic chemistry, namely compositional,
Compositional nomenclature
na
IR-1.5.3.2
lR
ec
substitutive, and additive nomenclature; they are described in more detail in Chapters IR5, IR-6 and IR-7 respectively. Additive nomenclature is perhaps the most generally
applicable in inorganic chemistry, but substitutive nomenclature may be applied in
appropriate areas. These two systems require knowledge of the constitution (connectivity)
of the compound or species being named. If only the stoichiometry or composition of a
compound is known or to be communicated, compositional nomenclature is used.
ov
isi
o
This term is used in the present recommendations to denote name constructions which are
based solely on the composition of the substances or species being named, as opposed to
systems involving structural information. One such construction is that of a generalised
stoichiometric name. The names of components which may be themselves elements or
composite entities (such as polyatomic ions) are listed with numerical prefixes giving the
PA
C
Pr
overall stoichiometry of the compound. If there are two or more components, they are
formally divided into two classes, the electropositive and the electronegative components.
In this respect, the names are like traditional salt names, although there is no implication
about the chemical nature of the species being named.
IU
Grammatical rules are then required to specify the ordering of components, the use of
numerical prefixes, and the proper endings for the names of the electronegative
components.
Examples:
1.
trioxygen, O3
2.
sodium chloride, NaCl
3.
phosphorus trichloride. PCl3
DRAFT 2 April 2004
Page 6 of 12
7
s
Substitutive nomenclature
da
IR-1.5.3.3
trisodium pentabismuthide, Na3Bi5
magnesium chloride hydroxide, MgCl(OH)
sodium cyanide, NaCN
ammonium chloride, NH4Cl
sodium acetate, NaO2CMe
tio
n
4.
5.
6.
7.
8.
om
me
n
Substitutive nomenclature is used extensively for organic compounds and is based on the
concept of a parent hydride modified by substitution of hydrogen atoms by atoms and/or
groups.21 (In particular it is used for naming organic ligands in the nomenclature of
coordination compounds, even though this is an overall additive system.)
ec
It is also used for naming compounds formally derived from the hydrides of certain
elements in groups 13-17 of the Periodic Table. Like carbon, these elements form chains
and rings which can have many derivatives, and the system avoids the necessity for
specifying the hydrogen atoms of the parent hydride.
lR
Rules are required to name parent compounds and substituents, to provide an order of
citation of substituent names, and to specify the positions of attachment of substituents.
2.
na
Examples:
1.
1,1-difluorotrisilane, SiH3SiH2SiHF2
trichlorophosphane, PCl3
Pr
Example:
3.
ov
isi
o
Replacement operations in which certain non-hydrogen atoms of parents are replaced by
different atoms or groups, e.g. the skeletal replacements leading to 'a' names in organic
chemistry (see Sections P-13.2.1 and P-51.3 of Ref 21), are usually considered as part of
substitutive nomenclature and are also used in certain parts of inorganic chemistry.
1,5-dicarba-closo-pentaborane(5), B3C2H5
PA
C
(CH replacing BH)
IU
Similarly, subtractive nomenclature can be applied in inorganic chemistry, particularly to
boron compounds.
Page 7 of 12
Example:
4.
4,5-dicarba-9-debor-closo-nonaborate(2–), [B6C2H8]2– (loss of BH)
DRAFT 2 April 2004
8
IR-1.5.3.4
Additive nomenclature
om
me
n
da
tio
n
s
Additive nomenclature treats a compound as a combination of a central atom or central
atoms with associated ligands. The particular additive system used for coordination
compounds (see Chapter IR-9) is sometimes known as coordination nomenclature
although it may be used for much wider classes of compounds, as demonstrated for
inorganic acids (Chapter IR-8) and organometallic compounds (Chapter IR-10) and for a
large number of simple molecules and ions named in Table IX. Another additive system is
well suited for naming chains and rings (Section IR-7.4).
Rules within these systems provide ligand names and guidelines for the order of citation of
ligand names and central atom names, designation of charge or unpaired electrons on
species, designation of stereochemistry, designation of point of ligation in complicated
ligands, etc.
Examples:
1.
PCl3, trichloridophosphorus
5.
6.
ec
lR
4.
[CoCl3(NH3)3], triamminetrichloridocobalt
H3SO4+ (= [SO(OH)3]+), trihydroxidooxidosulfur(1+)
_
[Pt(η2-C2H4)Cl3] , trichlorido(η2-ethene)platinate(1_)
HONH• , hydridohydroxidonitrogen(•)
ClSiH2SiH(Me)NSO,
na
2.
3.
2,2,3-trihydrido-3-methyl-4-azy-1-chlory-6-oxy-2,3-disily-5-sulfy-[6]catena
General naming procedures
isi
o
IR-1.5.3.5
ov
The three basic nomenclature systems may provide different but unequivocal names for a
given compound, as demonstrated for PCl3 above.
PA
C
Pr
The choice between the three depends on the class of inorganic compound under
consideration and the degree of detail one wishes to communicate. The following
examples further illustrate typical aspects that need to be considered before deciding on a
name.
IU
Examples:
1.
NO2
Would you like simply to specify a compound with this empirical formula, or a
compound with this molecular formula? Would you like to stress that it is a
radical? Would you like to specify the connectivity ONO?
2.
Al2(SO4)3·12H2O
DRAFT 2 April 2004
Page 8 of 12
9
H2P3O103
_
tio
n
3.
s
Would you like simply to indicate that this is a compound composed of
dialuminium trisulfate and water in the proportion 1:12, or would you like to
specify explicitly that it contains hexaaquaaluminium(3+) ions?
da
Would you like to specify that this is triphosphoric acid (as defined in Table IR8.1) from which three hydrons have been removed? Would you like to specify
where the two remaining hydrons are located?
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
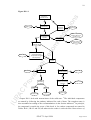
The flowchart shown in Figure IR-1.1 proposes general guidelines for naming compounds
and other species.
Page 9 of 12
DRAFT 2 April 2004
10
Figure IR-1.1
N
Y
Definite
stoichiometry?
N
Treat each
component
separatelyb
om
me
n
Y
Monoatomic or
homopolyatomic
species?
N
Molecule or
molecular ion?
N
Section IR-5.5
da
Chapter IR-11a
tio
n
s
Generalized addition
compound?
cf. Section IR-5.5
Y
Y
Table IX; Chapter IR-3;
Sections IR-5.3.2.2
and IR-5.3.3.2
N
Table IX;
Sections IR-5.3.2.3
and IR-5.3.3.3
Monoatomic?
Contains
metal?
na
lR
Section IR-5.4
ec
Y
Divide into electropositive
and electronegative
components and treat
each separatelyb
Y
Contains C?
C bonded to
transition metal?d
N
N
Decide:
substitutive
or additive
C bonded to
Group 1, 2 or 13-16
element?
isi
o
N
Y
PA
C
Pr
ov
Blue Bookc
substitutive
additive
N
Chapters
IR-7e or IR-8f
Chapter IR-9
Chapter IR-6
Y
Chapter IR-10
Y
Section IR-10.3
IU
a
Chapter IR-11 deals with nomenclature of the solid state. b The individual components
are named by following the pathway indicated for each of them. The complete name is
then assembled according to the recommendations in the Section indicated. c In principle,
the compound is outside the scope of this book. A few carbon compounds are named in
Tables IR-8.1, IR-8.2 and IX, but otherwise the reader is referred to the Nomenclature of
DRAFT 2 April 2004
Page 10 of 12
11
tio
n
s
Organic Compounds (the Blue Book). d C-bonded cyanides are treated as coordination
compounds, see Chapter IR-9. e The species may be named as a coordination-type
compound (Sections IR-7.1 to IR-7.3) or as a chain or ring (Section IR-7.4). f For
inorganic acids.
IR-1.6
NOMENCLATURE RECOMMENDATIONS IN OTHER AREAS OF CHEMISTRY
REFERENCES
6.
7.
8.
ec
lR
PA
C
9.
na
4.
5.
isi
o
3.
L.B. Guyton de Morveau, J. Phys., 19, 310 (1782); Ann. Chim. Phys., 1, 24 (1798).
L.B. Guyton de Morveau, A.L. Lavoisier, C.L. Berthollet and A.F. de Fourcroy,
Méthode de Nomenclature Chimique, Paris, 1787.
A.L. Lavoisier, Traité Elémentaire de Chimie, Third Edn, Deterville, Paris, 1801,
Vol. I, pp. 70-81, and Vol. II.
J.J. Berzelius, J. Phys., 73, 248 (1811).
A. Werner, Neuere Anschauungen auf den Gebiete der Anorganischen Chemie,
Third Edn, Vieweg, Braunschweig, 1913, pp. 92-95.
Bull. Soc. Chem. (Paris), 3(7), XIII (1892).
W.P. Jorissen, H. Bassett, A. Damiens, F. Fichter and H. Remy, Ber. Dtsch. Chem.
Ges. A, 73, 53 (1940); J. Chem. Soc., 1404 (1940); J. Am. Chem. Soc., 63, 889
(1941).
Nomenclature of Inorganic Chemistry, 1957 Report of CNIC, IUPAC, Butterworths
Scientific Publications, London, 1959; J. Am. Chem. Soc., 82, 5523 (1960).
Nomenclature of Inorganic Chemistry. Definitive Rules 1970, Second Edn,
Butterworths, London, 1971.
How to Name an Inorganic Substance. A Guide to the Use of Nomenclature of
Inorganic Chemistry, Pergamon Press, Oxford, 1977.
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell
Scientific Publications, Oxford, 1990.
Nomenclature of Polyanions, Pure Appl. Chem., 59, 1529 (1987).
Nomenclature of Tetrapyrroles, Pure Appl. Chem., 59, 779 (1987); Eur. J.
Biochem., 108, 1 (1988).
ov
1.
2.
Pr
IR-1.7
om
me
n
da
Inorganic chemical nomenclature, as inorganic chemistry itself, does not develop in
isolation from other fields, and those working in interdisciplinary areas will find useful
IUPAC texts on the general principles of chemical nomenclature22 as well as the specific
topics of organic,21 biochemical,23 analytical24 and macromolecular chemistry.25 Other
IUPAC publications include a glossary of terms in bioinorganic chemistry,26 a
compendium of chemical terminology27 and quantities, units and symbols in physical
chemistry.28 Other texts concerning chemical nomenclature are given in Ref. 29.
10.
IU
11.
Page 11 of 12
12.
13.
DRAFT 2 April 2004
12
22.
23.
24.
25.
26.
27.
28.
s
tio
n
da
Pergamon Press, Oxford, 1979; A Guide to IUPAC Nomenclature of Organic
Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford,
1993; Pure Appl. Chem., 71, 1327 (1999)].
Principles of Chemical Nomenclature, A Guide to IUPAC Recommendations,
Blackwell Scientific Publications, Oxford, 1998.
Biochemical Nomenclature and Related Documents, for IUBMB, Portland Press
Ltd., London, 1992.
Compendium of Analytical Nomenclature, Definitive Rules, 1997, Third Edn.,
Blackwell Scientific Publications, Oxford, 1998.
Compendium of Macromolecular Nomenclature, Blackwell Scientific Publications,
Oxford, 1991.
Glossary of Terms used in Bioinorganic Chemistry, Pure Appl. Chem., 69, 1251
(1997).
Compendium of Chemical Terminology, IUPAC Recommendations, Second Edn,
Blackwell Scientific Publications, Oxford, 1997.
Quantities, Units and Symbols in Physical Chemistry, Second Edn, Blackwell
Scientific Publications, Oxford, 1993. (Update not yet published).
Nomenclature of Coordination Compounds, T.E. Sloan, Vol. 1, Chapter 3,
Comprehensive Coordination Chemistry, Pergamon, 1987; Inorganic Chemical
Nomenclature, Principles and Practice, B.P. Block, W.H. Powell and W.C.
Fernelius, American Chemical Society, Washington, DC, 1990; Chemical
Nomenclature, K.J. Thurlow, Kluwer Academic Pub., 1998.
IU
PA
C
29.
om
me
n
21.
ec
20.
lR
19.
na
18.
isi
o
16.
17.
ov
15.
Nomenclature of Inorganic Chains and Ring Compounds, Pure Appl. Chem., 69,
1659 (1997).
Nomenclature and Terminology of Graphite Intercalation Compounds, Pure Appl.
Chem., 66, 1893 (1994).
Isotopically Modified Compounds, Pure Appl. Chem., 53, 1887 (1981).
The Nomenclature of Hydrides of Nitrogen and Derived Cations, Anions, and
Ligands, Pure Appl. Chem., 54, 2545 (1982).
Nomenclature for Regular Single-strand and Quasi Single-strand Inorganic and
Coordination Polymers, Pure Appl. Chem. 57, 149 (1985).
Nomenclature of Inorganic Chemistry II, Recommendations 2000, Royal Society of
Chemistry, 2001.
Nomenclature of Organometallic Compounds of the Transition Elements, Pure
Appl. Chem., 71, 1557 (1999).
Title of New Blue Book needed. [See also, Nomenclature of Organic Chemistry,
Pr
14.
DRAFT 2 April 2004
Page 12 of 12
1
IR-2
Grammar (March 2004)
tio
n
s
CONTENTS
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
IR-2.1 Introduction
IR-2.2 Enclosing marks
IR-2.2.1 General
IR-2.2.2 Square brackets
IR-2.2.2.1 Use in formulae
IR-2.2.2.2 Use in names
IR-2.2.3 Parentheses
IR-2.2.3.1 Use in formulae
IR-2.2.3.2 Use in names
IR-2.2.4 Braces
IR-2.3 Hyphens, plus and minus signs, 'em' dashes and bond indicators
IR-2.3.1 Hyphens
IR-2.3.2 Plus and minus signs
IR-2.3.3 'Em' dashes
IR-2.3.4 Special bond indicators for line formulae
IR-2.4 Solidus
IR-2.5 Dots, colons, commas and semicolons
IR-2.5.1 Dots
IR-2.5.2 Colons
IR-2.5.3 Commas
IR-2.5.4 Semicolons
IR-2.6 Spaces
IR-2.7 Elisions
IR-2.8 Numerals
IR-2.8.1 Arabic numerals
IR-2.8.2 Roman numerals
IR-2.9 Italic letters
IR-2.10 Greek alphabet
IR-2.11 Asterisks
IR-2.12 Primes
IR-2.13 Multiplicative prefixes
IR-2.14 Locants
IR-2.14.1 Introduction
IR-2.14.2 Arabic numerals
IR-2.14.3 Lower case letters
IR-2.15 Priorities
Page 1 of 33
DRAFT 2 April 2004
INTRODUCTION
ec
IR-2.1
om
me
n
da
tio
n
IR-2.15.1 Introduction
IR-2.15.2 Electronegativity criterion
IR-2.15.3 Alphabetical order
IR-2.15.4 Element priority sequence
IR-2.15.5 Other priority sequences
IR-2.15.5.1 Organic priority orders
IR-2.15.5.2 Priority of ligand types
IR-2.15.5.3 Priorities in salt formulae and names
IR-2.15.5.4 Isotope labelling and modification
IR-2.15.5.5 Stereochemical priorities
IR-2.15.5.6 Priority sequences of punctuation marks
IR-2.16 Affixes (prefixes, suffixes and infixes)
IR-2.17 Final remarks
IR-2.18 References
s
2
lR
Chemical nomenclature may be considered to be a language. As such, it consists of words
and it should obey the rules of syntax.
isi
o
na
In the language of chemical nomenclature, the simple names of atoms are the words. As
words are assembled to form a sentence, so names of atoms are assembled to form names
of chemical compounds. Syntax is the set of grammatical rules for building sentences out
of words. In nomenclature, syntax includes the use of symbols, such as dots, commas and
hyphens, the use of numbers for appropriate reasons in given places, and the order of
citation of various words, syllables and symbols.
PA
C
Pr
ov
Generally nomenclature systems require a root on which to construct the name. This root
can be an element name (e.g. 'cobalt' or 'silicon') for use in additive nomenclature, or can
be derived from an element name (e.g. 'sil' from 'silicon', 'plumb' from 'plumbum' for lead)
and elaborated to yield a parent hydride name (e.g. 'silane' or 'plumbane') for use in
substitutive nomenclature.
IU
Names are constructed by joining other units to these roots. Among the most important
units are affixes. These are syllables or numbers added to words or roots and can be
suffixes, prefixes or infixes according to whether they are placed after, before or within a
word or root.
DRAFT 2 April 2004
Page 2 of 33
3
tio
n
s
Suffixes (endings) are of many different kinds (Table III)*, each of which conveys specific
information. The following examples illustrate particular uses. They may specify the
degree of unsaturation of a parent compound in substitutive nomenclature: hexane,
hexene; and phosphane, diphosphene, diphosphyne. Other endings indicate the nature of
the charge carried by the whole compound; cobaltate refers to an anion. Further suffixes
can indicate that a name refers to a group, as in hexyl.
om
me
n
da
Prefixes indicate, for example, substituents in substitutive nomenclature, as in the name
chlorotrisilane, and ligands in additive nomenclature, as in the name aquacobalt. The
prefixes can also be numbers to express specific information such as a point of attachment,
e.g. 2-chlorotrisilane, or they can be multiplicative prefixes (Table IV) to indicate the
number of constituents or ligands, e.g. hexaaquacobalt.
ec
Prefixes may be used to describe the structural types or other structural features of species;
geometrical and structural prefixes are listed in Table V. Other devices may be used to
complete the description of the compound. These include the charge number to indicate
the ionic charge, e.g. hexaaquacobalt(2+), and, alternatively, the oxidation number to
indicate the oxidation state of the central atom, e.g. hexaaquacobalt(II).
isi
o
na
lR
The designation of central atom and ligands, generally straightforward in mononuclear
complexes, is more difficult in polynuclear compounds where there are several central
atoms in the compound to be named, e.g. in polynuclear coordination compounds,
polyoxoanions, and chain and ring compounds. In each case, a priority order or hierarchy
has to be established. A hierarchy of functional groups is an established feature of
substitutive nomenclature; Table VI shows one of the priority sequences used in additive
nomenclature.
IR-2.2
PA
C
Pr
ov
The purpose of this Chapter is to guide the users of nomenclature in building the name or
formula of an inorganic compound and to help them verify that the derived name or
formula fully obeys the accepted principles. The various devices used in names (or
formulae) are described successively below, together with their meanings and fields of
application.
IU
IR-2.2.1
ENCLOSING MARKS
General
Chemical nomenclature employs three types of enclosing mark, namely: braces { }, square
brackets [ ], and parentheses ( ).
*
Page 3 of 33
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
4
tio
n
s
In formulae, square brackets, parentheses and braces are used in the following nesting
order: [], [( )], [{( )}], [({( )})], [{({( )})}], etc. Square brackets are normally used only to
enclose formulae; parentheses and braces are then used alternately (see also Sections IR4.2.3 and IR-9.2.3.2).
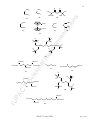
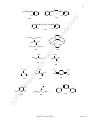
[Rh3Cl(µ-Cl)(CO)3{µ3-Ph2PCH2P(Ph)CH2PPh2}2]+
Ph
Ph2P
P
om
me
n
Example:
1.
da
In names, the nesting order is: (), [()], {[()]}, ({[()]}), etc. This ordering is that used in
substitutive nomenclature (see Section P-16.4.3.2 of Ref. 1). (See also, Section IR-9.2.2.3
for the use of enclosing marks with ligand names).
+
CO
OC
Rh
Rh
Cl
Cl
Rh
C
ec
O
P
PPh2
lR
Ph2P
PPh2
Ph
na
tricarbonyl-1κC,2κC,3κC-µ-chlorido-1:2κ2Cl-chlorido-3κCl-bis{µ3-
isi
o
bis[(diphenylphosphanyl)-1κP':3κP''-methyl]phenylphosphane2κP}trirhodium(1+)
Square brackets
IR-2.2.2.1
Use in formulae
ov
IR-2.2.2
Pr
Square brackets are used in formulae in the following ways.
(a) To enclose the whole coordination entity of a neutral (formal) coordination compound.
PA
C
Examples:
1.
[Fe(η5-C5H5)2]
IU
(for use of the symbol η see Sections IR-9.2.4.3 and IR-10.2.5.1)
2.
3.
[Pt(η2-C2H4)Cl2(NH3)]
[PH(O)(OH)2]
DRAFT 2 April 2004
Page 4 of 33
5
tio
n
s
No numerical subscript should follow the square bracket used in this context. For example,
where the molecular formula is double the empirical formula, this should be indicated
inside the square bracket.
Example:
4.
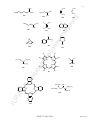
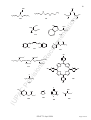
CH2
Cl
Pt
H2 C
Pt
CH2
Cl
om
me
n
Cl
da
Cl
H2 C
[{Pt(η2-C2H4)Cl(µ-Cl)}2] is more informative than [Pt2(η2-C2H4)2Cl4]; the
representation [Pt(η2-C2H4)Cl2]2 is incorrect.
ec
(b) To enclose the coordination entity of a charged (formal) coordination compound. In
this case, the superscript showing the charge appears outside the square bracket as do any
subscripts indicating the number of ions in the salt.
7.
8.
9.
10.
ov
11.
[Al(OH)(OH2)5]2+
_
[Pt(η2-C2H4)Cl3]
Ca[AgF4]2
[Co(NH3)5(N3)]SO4
_
[S2O5]2
_
[PW12O40]3
na
6.
_
[BH4]
isi
o
5.
lR
Examples:
PA
C
Pr
(c) In a salt comprising both cationic and anionic coordination entities, each ion is
separately enclosed in square brackets. (The cation is placed before the anion and no
individual charges are shown). Any subscripts indicating the number of complex ions in
the salt are shown outside the square brackets.
Examples:
12. [Co(NH3)6][Cr(CN)6]
IU
_
(comprising the ions [Co(NH3)6]3+ and [Cr(CN)6]3 )
Page 5 of 33
13.
[Co(NH3)6]2[Pt(CN)4]3
_
(comprising the ions [Co(NH3)6]3+ and [Pt(CN)4]2 )
DRAFT 2 April 2004
6
(d) To enclose structural formulae.
tio
n
s
Example:
14.
da
+
om
me
n
Mo(CO)3
[Mo(η7-C7H7)(CO)3]+
(Mg)[Cr2]O4
lR
Example:
15.
ec
(e) In solid state chemistry, to indicate an atom or a group of atoms in an octahedral site.
(See Section IR-11.4.3)
H2[15N]NH2
isi
o
Example:
16.
na
(f) In specifically labelled compounds (see also Section II-2.4.2.2 of Ref. 2).
Note that this distinguishes the specifically labelled compound from the isotopically
substituted compound H215NNH2.
[18O,32P]H3PO4
Pr
Example:
17.
ov
(g) In selectively labelled compounds (see also Section II-2.4.3.2 of Ref. 2).
PA
C
(h) To indicate repeating units in chain compounds.
Example:
18.
IU
IR-2.2.2.2
SiH3[SiH2]8SiH3
Use in names
Square brackets are used in names in the following ways.
DRAFT 2 April 2004
Page 6 of 33
7
[13C][Fe(CO)5]
2.
[2H1,15N]ammonia
[13C]pentacarbonyliron
om
me
n
For more details, see Section II-2.4 of Ref. 2.
da
Examples:
1.
[15N]H2[2H]
tio
n
s
(a) In specifically and selectively labelled compounds the nuclide symbol is placed in
square brackets before the name of the part of the compound that is isotopically modified.
(Compare with the use of parentheses for isotopically substituted compounds in Section
IR-2.2.3.2, and also see Sections II-2.4.2.3 and II-2.4.3.3 of Ref. 2).
(b) When naming organic ligands and organic parts of coordination compounds the use of
square brackets obeys the principles of substitutive nomenclature.1
Parentheses
IR-2.2.3.1
Use in formulae
lR
IR-2.2.3
ec
(c) In chain and ring nomenclature, square brackets are used to enclose the nodal
descriptor (Section IR-7.4.2 and Chapter II-5 of Ref. 2).
Parentheses are used in formulae in the following ways.
isi
o
na
(a) To enclose sets of identical groups of atoms (the entity may be an ion, substituent
group, or molecule). Usually a multiplicative subscript follows the closing parenthesis. In
the case of common ions such as nitrate and sulfate parentheses are recommended but not
mandatory.
B3H3(NMe)3
[Ni(CO)4]
_
_
[NO3] or NO3
[FeH(H2)(Ph2PCH2CH2PPh2)2]+
Pr
2.
3.
ov
Examples:
1.
Ca3(PO4)2
IU
PA
C
4.
5.
Page 7 of 33
(b) To enclose the formula of a moiety which is an atom or set of atoms forming a neutral
or charged ligand in a coordination compound. The purpose is to separate the ligands from
each other or from the remaining part of the molecule in order to avoid ambiguity.
Parentheses may be used even if a multiplicative suffix is not needed.
Example:
7.
8.
[Co(NH3)5(ONO)]SO4 or [Co(NH3)5(ONO)][SO4]
[Co(NH3)5(ONO)][PF6]2
DRAFT 2 April 2004
8
[Co(en)3]3+
tio
n
Example:
9.
s
(c) To enclose the abbreviation of a ligand name in formulae. (Recommended ligand
abbreviations are given in Tables VII and VIII. See also Sections IR-4.4.4 and IR-9.2.3.4).
(d) To enclose the superscripted radical dot and its multiplier for polyradicals, in order to
da
avoid ambiguity in relation to multiplying the charge symbol.
Example:
NO(2•)
_
om
me
n
10.
(e) In solid state chemistry, to enclose symbols of atoms occupying the same type of site in
a random fashion. The symbols themselves are separated by a comma, with no space.
K(Br,Cl)
ec
Example:
11.
Example:
12.
(Mg)[Cr2]O4
lR
(f) In solid state chemistry, to indicate an atom or a group of atoms in a tetrahedral site.
na
(g) To indicate the composition of a non-stoichiometric compound.
isi
o
Examples:
13. Fe3xLi4-xTi2(1-x)O6
14. LaNi5Hx
(x = 0.35)
(0 < x < 6.7)
(CrMgVMgCrMg)x
Pr
Example:
15.
ov
(h) In the Kröger-Vink notation (see Section IR-11.4), to indicate a complex defect.
PA
C
(i) For crystalline substances, to indicate the type of crystal formed (see Chapter IR-11).
IU
Examples:
16. ZnS(c)
17. AuCd (CsCl type)
(j) In optically active compounds, to enclose the signs of rotation or the symbols for
absolute configuration.
DRAFT 2 April 2004
Page 8 of 33
9
s
(+)589-[Co(en)3]Cl3
(2R,3S)-1,2,3-ClSiH2SiHClSiHClSiH2SiH3
Example:
20.
(OC-6-22)-[Co(NH3)3(NO2)3]
da
(k) To enclose configuration indices (see Section IR-9.3.3.2)
tio
n
Example:
18.
19.
om
me
n
(l) For crystalline substances, to indicate the type of crystal formed (see Chapter IR-11).
(m) In polymers, the repeating unit is enclosed in strike-through parentheses, with the dash
superimposed on the parentheses representing the bond.3
Example:
21.
Use in names
ec
IR-2.2.3.2
(S)n
lR
Parentheses are used in names in the following ways.
[CuCl2(NH2Me)2]
dichloridobis(methylamine)copper(II)
isi
o
Example:
1.
na
(a) Following multiplicative prefixes such as bis and tris, unless a nesting order is to be
used (see Section IR-2.2.1).
Na[B(NO3)4]
sodium tetranitratoborate(III), or
sodium tetranitratoborate(1_)
Pr
Example:
2.
ov
(b) To enclose oxidation and charge numbers.
(c) For radicals, to enclose the radical dot, and the charge number if appropriate.
PA
C
Examples:
3.
ClOO•
4.
Cl2•−
chloridodioxygen(•)
dichloride(•1−)
IU
(d) To enclose stoichiometric ratios for addition compounds and clathrates.
Page 9 of 33
Example:
5.
8H2S.46H2O
hydrogen sulfide—water (8/46)
DRAFT 2 April 2004
10
[Mn2(CO)10]
bis(pentacarbonylmanganese)(Mn—Mn)
da
(f) To enclose stereochemical descriptors (see Section IR-9.3.2)
Cl
H3N
Cl
Co
Cl
NH3
[CoCl3(NH3)3]
om
me
n
Example:
7.
H3N
tio
n
Example:
6.
s
(e) To enclose italic letters representing bonds between two (or more) metal atoms in
coordination compounds.
ec
(OC-6-22)-triamminetrichloridocobalt(III)
lR
(g) To enclose inorganic ligand names, such as (triphosphato), which contain numerical
prefixes.
Example:
_
[Pt(η2-C2H4)Cl3]
trichlorido(η2-ethene)platinate(II)
ov
8.
isi
o
na
(h) To enclose organic ligand names whether they are neutral or not, or whether they are
substituted or not, e.g. (benzaldehyde), (benzoato), etc. It may be necessary to use a higher
order of enclosing marks if the ligand names themselves include parentheses.
Pr
(i) In isotopically substituted compounds, the appropriate nuclide symbol(s) is placed in
parentheses before the name of the part of the compound that is isotopically substituted
(see Section II-2.3.3 of Ref. 2). Compare with the use of square brackets for specifically
and selectively labelled compounds in Section IR-2.2.2.2(a).
PA
C
Example:
9.
H3HO
(3H1)water
IU
(j) To enclose the number of hydrogen atoms in boron compounds.
Example:
10.
B6H10
hexaborane(10)
DRAFT 2 April 2004
Page 10 of 33
11
s
(k) In hydrogen names (Section IR-8.4), to enclose the part of the name following the
word hydrogen.
Example:
hydrogen(nonadecaoxidohexamolybdate)(1_)
Braces
da
IR-2.2.4
_
[HMo6O19]
tio
n
11.
om
me
n
Braces are used in names and formulae within the hierarchical sequence outlined and
exemplified in Section IR-2.2.1.
IR-2.3
HYPHENS, PLUS AND MINUS SIGNS, 'EM' DASHES AND BOND INDICATORS
IR-2.3.1
Hyphens
Hyphens are used in formulae and in names. Note that there is no space on either side of a
hyphen.
lR
ec
(a) To separate symbols such as µ (mu), η (eta) and κ (kappa) from the rest of the formula
or name.
Example:
1.
isi
o
na
(b) To separate geometrical or structural and stereochemical designators such as cyclo,
catena, triangulo, quadro, tetrahedro, octahedro, closo, nido, arachno, cis and trans from
the rest of the formula or name. In dealing with aggregates or clusters, locant designators
are similarly separated.
Pr
ov
Br
C
Co
(CO)3
Co(CO)3
PA
C
(OC)3Co
µ3-(bromomethylidyne)-cyclo-tris(tricarbonylcobalt)(3 Co—Co)
IU
(c) To separate locant designators from the rest of the name.
Example:
2.
SiH2ClSiHClSiH2Cl
1,2,3-trichlorotrisilane
(d) To separate the labelling nuclide symbol from its locant in the formula of a selectively
labelled compound.
Page 11 of 33
DRAFT 2 April 2004
12
Example:
3.
tio
n
(e) To separate the name of a bridging ligand from the rest of the name.
Example:
4.
Fe
Fe
OC
C
O
CO
C
O
[Fe2(µ-CO)3(CO)6]
IR-2.3.2
da
CO
CO
om
me
n
OC
O
C
OC
s
[1-2H1;2]SiH3OSiH2OSiH3
tri-µ-carbonyl-bis(tricarbonyliron)
Plus and minus signs
ec
The signs + and _ are used to indicate the charge on an ion in a formula or name.
Examples:
lR
_
Cl
2.
Fe3+
3.
[SO4]2
4.
_
[Co(CO)4]
tetracarbonylcobaltate(1_)
isi
o
_
na
1.
IR-2.3.3
(+)589[Co(en)3]3+
(+)589-tris(ethane-1,2-diamine)cobalt(3+)
Pr
Example:
5.
ov
They can also indicate the sign of optical rotation in the formula or name of an optically
active compound.
'Em' dashes
PA
C
'Em' dashes are used in formulae only when the formulae are structural. (The less precise
term 'long dashes' was used in Ref. 4).
IU
In names, 'em' dashes are used in two ways.
(a) To indicate metal-metal bonds in polynuclear compounds. They separate the italicized
symbols of the bond partners which are contained in parentheses at the end of the name.
Example:
1.
[Mn2(CO)10]
bis(pentacarbonylmanganese)(Mn—Mn)
DRAFT 2 April 2004
Page 12 of 33
13
(b) To separate the molecular constituents in names of addition compound.
Examples:
da
Special bond indicators for line formulae
may be used in line formulae to indicate bonds
om
me
n
and
The structural symbols
between non-adjacent atom symbols.
Examples:
1.
Me
Me
P
Ni
lR
[Ni(S=PMe2)(η5-C5H5)]
ec
S
isi
o
Ph2P
na
2.
(OC)4Mn
Mo(CO)3
Et3P
IU
PA
C
Pr
3.
ov
[(CO)4MnMo(CO)3(η5-C5H4PPh2)]
Page 13 of 33
tio
n
cadmium sulfate—water (3/8)
2CHCl3.4H2S.9H2O chloroform_hydrogen sulfide—water (2/4/9)
3.
IR-2.3.4
s
3CdSO4.8H2O
2.
Cl
Pt
NMe2
Me2N
Pt
Cl
PEt3
[(Et3P)ClPt(Me2NCH2CHCHCH2NMe2)PtCl(PEt3)]
DRAFT 2 April 2004
14
4.
H
C
PPh2
s
Ph2P
(OC)2Fe
tio
n
H
Fe(CO)3
IR-2.4
om
me
n
[(OC)3Fe(µ-Ph2PCHPPh2)FeH(CO)3]
da
C
O
SOLIDUS
The solidus (/) is used in names of addition compounds to separate the arabic numerals
which indicate the proportions of individual constituents in the compound.
Examples:
BF3.2H2O
boron trifluoride—water (1/2)
2.
BiCl3.3PCl5
bismuth trichloride—phosphorus pentachloride (1/3)
ec
1.
DOTS, COLONS, COMMAS AND SEMICOLONS
IR-2.5.1
Dots
na
lR
IR-2.5
Dots are used in formulae in various positions.
isi
o
(a) As right superscripts they indicate unpaired electrons in radicals (see Section IR-4.6.2).
ov
Examples:
1.
HO•
2.
O22•
Pr
(b) As right superscripts in the Kröger-Vink notation of sold-state chemistry, they indicate
the unit of positive effective charge (see Section IR-11.4.4).
IU
PA
C
Example:
3.
x
•
′ x
LiLi,1-2
x MgLi,xVLi,x ClCl
(c) Centre dots in formulae of hydrates, addition compounds, double salts, and double
oxides separate the individual constituents. The dot is written in the centre of the line to
distinguish it from a full stop (period).
Examples:
4.
ZrCl2O.8H2O
DRAFT 2 April 2004
Page 14 of 33
15
NH3.BF3
6.
CuCl2.3Cu(OH)2
7.
Ta2O5.4WO3
tio
n
s
5.
Examples:
8.
ClO•
9.
Cl2•−
om
me
n
IR-2.5.2
oxidochlorine(•)
dichloride(•1−)
da
Dots are used in names of radicals to indicate the presence of unpaired electrons.
Colons
Colons are used in names in the following ways.
Example:
1.
ec
(a) In coordination compounds, to separate the ligating atoms of a ligand which bridges
central atoms.
[{Co(NH3)3}2(µ-NO2)(µ-OH)2]3+
lR
di-µ-hydroxido-µ-nitrito-κN:κO-bis(triamminecobalt)(3+)
na
(See Sections IR-9.2.4.2 and IR-10.2.3.3 for the use of κ, and Sections IR-9.2.5.2
and IR-10.2.3.1 for the use of µ)
ov
Example:
2.
isi
o
(b) In boron compounds, to separate the sets of locants of boron atoms which are
connected by bridging hydrogen atoms.
Pr
H
5B
PA
C
H
B 2
H
H
H
SiH3
1
B
B
4
H
B
3
H
H
IU
1-silyl-2,3:2,5:3,4:4,5-tetra-µH-pentaborane(9)
Page 15 of 33
(c) In chains and rings nomenclature, to separate nodal descriptors of individual modules
of an assembly (see Section IR-7.4.2).
DRAFT 2 April 2004
16
IR-2.5.3
Commas
s
Commas are used in the following ways.
Example:
1.
tio
n
(a) To separate locants.
da
SiH2ClSiHClSiH2Cl 1,2,3-trichlorotrisilane
Example:
2.
om
me
n
(b) To separate the symbols of the ligating atoms of a polydentate chelating ligand.
cis-bis(glycinato-N,O)platinum
(c) In solid state chemistry, to separate symbols of atoms occupying the same type of site
in a random fashion.
Example:
3.
ec
(Mo,W)nO3n-1
lR
(d) To separate oxidation numbers in a mixed valence compound.
N
N
5+
Ru(NH3)5
isi
o
(H3N)5Ru
na
Example:
4.
[(NH3)5Ru(µ-pyz)Ru(NH3)5]5+
ov
µ-pyrazine-bis(pentaammineruthenium)(II,III)
Pr
(e) To separate symbols of labelled atoms in selectively labelled compounds.
[18O,32P]H3PO4
[18O,32P]phosphoric acid
PA
C
Example:
5.
IR-2.5.4
Semicolons
IU
Semicolons are used in the following ways.
(a) In the names of coordination compounds, to order locants separated by commas, as in
the kappa convention. (See Sections IR-9.2.4.2 and IR-10.2.3.3).
DRAFT 2 April 2004
Page 16 of 33
17
Example:
tio
n
1.
s
(1)
(2)
[Cu(bpy)(H2O)(µ-OH)2Cu(bpy)(SO4)]
da
aqua-1κO-bis(2,2'-bipyridine)-1κ2N1,N1';2κ2N1,N1'-di-µ-hydroxido[sulfato(2_)2κO]dicopper(II)
Example:
2.
IR-2.6
[1-2H1;2]SiH3OSiH2OSiH3
SPACES
om
me
n
(b) To separate the subscripts in order to indicate the number of possible positions in
selectively labelled compounds.
ec
In inorganic nomenclature, spaces are used in names in the following ways in English; the
rules may differ in other languages. Spaces are never used in formulae.
NaCl
sodium chloride
NaTl(NO3)2 sodium thallium(I) dinitrate
na
Examples:
1.
2.
lR
(a) To separate the names of ions in salts.
P4O10
ov
Example:
3.
isi
o
(b) In binary compounds, to separate the electropositive part from the electronegative part.
tetraphosphorus decaoxide
Pr
(c) To separate the arabic numeral from the symbols of central atoms written in italics
between parentheses at the end of the name of a polynuclear compound.
IU
PA
C
Example:
4.
(d) In addition compounds, to separate constituent proportions from the remainder of the
name.
Example:
5.
Page 17 of 33
[Os3(CO)12] cyclo-tris(tetracarbonylosmium)(3 Os—Os)
3CdSO4.8H2O
cadmium sulfate—water (3/8)
DRAFT 2 April 2004
18
Example:
6.
TiO2(r) (brookite type)
ELISIONS
da
IR-2.7
tio
n
s
(e) In solid state chemistry, to separate name and structural type.
om
me
n
In general, in compositional and additive nomenclature no elisions are made when using
numerical prefixes.
Example:
1.
2.
3.
tetraaqua and not tetraqua
monoooxygen and not monoxygen
tetraarsenic hexaoxide
IR-2.8
NUMERALS
IR-2.8.1
Arabic numerals
na
lR
ec
However, monoxide, rather than monooxide (preferred), is an allowed exception through
general use.
isi
o
Arabic numerals are crucially important in nomenclature; their place in a formula or name
is especially significant.
ov
They are used in formulae in many ways.
Pr
(a) As right subscripts, to indicate the number of individual constituents (atoms or groups
of atoms). Unity is not indicated.
IU
PA
C
Examples:
1.
2.
CaCl2
[Co(NH3)6]Cl3
(b) As a right superscript, to indicate the charge number. Unity is not indicated.
Examples:
3.
Cl
_
DRAFT 2 April 2004
Page 18 of 33
19
tio
n
s
NO+
Cu2+
[Al(H2O)6]3+
4.
5.
6.
da
(c) To indicate the composition of addition or non-stoichiometric compounds. The
numeral is written on the line before the molecular formula of each constituent except that
unity is omitted.
7.
Na2CO3.10H2O
8.
8WO3.9Nb2O5
om
me
n
Examples:
(d) To designate the mass number and/or the atomic number of nuclides represented by
their symbols. The mass number is written as a left superscript, and the atomic number as
3
1
O
H
na
10.
18
8
lR
Examples:
9.
ec
a left subscript.
[Fe(η5-C5H5)2]
ov
Example:
11.
isi
o
(e) As a right superscript to the symbol η, to indicate the hapticity of a ligand (see
Sections IR-9.2.4.3 and IR-10.2.5.1).
Pr
Arabic numerals are also used as locants in names (see Section IR-2.14.2), and in the
following ways.
PA
C
(a) To indicate the number of metal-metal bonds in polynuclear compounds.
Example:
IU
12.
Page 19 of 33
DRAFT 2 April 2004
20
O
C
s
Ni
tio
n
Ni
Ni
om
me
n
[{Ni(η5-C5H5)}3(µ3-CO)2]
da
C
O
di-µ3-carbonyl-cyclo-tris(cyclopentadienylnickel)(3 Ni—Ni)
(b) To indicate the charge number.
pentaamminechloridocobalt(2+)
tetrachloridoaluminate(1_)
lR
14.
[CoCl(NH3)5]2+
_
[AlCl4]
ec
Example:
13.
na
Note that the number '1' must be included in order to avoid ambiguity in relation to
symbols for optical rotation [see Section IR-2.2.3.1(j)].
I
Pr
PtMe3
Me3Pt
I
Me3Pt
I
I
PtMe3
ov
Example:
14.
isi
o
(c) As a right subscript to the symbol µ, to indicate bridging multiplicity.
tetra-µ3-iodidotetrakis[trimethylplatinum(IV)]
PA
C
[{Pt(µ3-I)Me3}4]
IU
(d) In the nomenclature of boron compounds (see Chapter IR-6.2.3), to indicate the
number of hydrogen atoms in the parent borane molecule. The arabic numeral is enclosed
in parentheses immediately following the name.
Examples:
15.
16.
B2H6
B10H14
diborane(6)
decaborane(14)
DRAFT 2 April 2004
Page 20 of 33
21
s
(e) As a right superscript to the symbol κ, to indicate the number of donor atoms of a
particular type bound to a central atom (see Section IR-9.2.4.2).
tio
n
(f) As a right superscript to the symbol η, to indicate the hapticity of a ligand. (See
Sections IR-9.2.4.3 and IR-10.2.5.1).
da
(g) In polynuclear structures, arabic numerals are part of the CEP descriptor6 used to
identify polyhedral shapes. (See also Section IR-9.2.5.6).
om
me
n
(h) In addition compounds, to indicate the numbers of molecules of the constituents.
Example:
17.
8H2S.46H2O
hydrogen sulfide—water (8/46)
Roman numerals
lR
IR-2.8.2
λ5-iodane
IH5
na
Example:
18.
ec
(i) As a right superscript, to indicate the non-standard bonding number in the λ
convention. (See Section IR-6.2.1).
1.
[CoIICoIIIW12O42]7
_
[MnVIIO4]
FeIIFeIII2O4
_
Pr
2.
3.
ov
Examples:
isi
o
Roman numerals are used in formulae as right superscripts to designate the oxidation
number.
IU
PA
C
In names they indicate the oxidation number of an atom, and are enclosed in parentheses
immediately following the name of the atom being qualified.
IR-2.9
Example:
4.
[Fe(H2O)6]2+
hexaaquairon(II)
ITALIC LETTERS
Italic letters are used in names as follows.
Page 21 of 33
DRAFT 2 April 2004
22
tio
n
s
(a) For geometrical and structural prefixes such as cis, cyclo, catena, triangulo and nido.
(see Table V).
[Mn2(CO)10]
bis(pentacarbonylmanganese)(Mn—Mn)
om
me
n
Example:
1.
da
(b) To designate symbols of metal atoms bonded to other metal atoms in polynuclear
compounds.
(c) In double oxides and hydroxides when the structural type is to be indicated.
Example:
2.
MgTiO3
magnesium titanium trioxide (ilmenite type)
ec
(d) In coordination compounds, to designate the symbols of the atom or atoms of a ligand
(usually polydentate) to which the metal is bound, whether the kappa convention is used
or not. (See Section IR-9.2.4.4).
lR
Example:
3.
O
O
na
C
isi
o
H2C
N
H2
O
Pt
N
H2
O
C
CH2
ov
cis-bis(glycinato-N,O)platinum
Pr
(e) In solid state chemistry, in Pearson and crystal system symbols. (See Sections IR-3.5.3
and IR-11.5).
PA
C
(f) In coordination compounds, italicized capital letters designate polyhedral symbols.
(See Section IR-9.3.2.1).
IU
Example:
4.
DRAFT 2 April 2004
Page 22 of 33
23
Cl
H3N
Cl
tio
n
H3N
s
Co
Cl
NH3
(OC-6-22)-triamminetrichloridocobalt(III)
da
[CoCl3(NH3)3]
Examples:
5.
(HBO2)n
Fen+
6.
GREEK ALPHABET
ec
IR-2.10
om
me
n
(g) Italic letters are also used to represent numbers, especially in formulae, where the
numbers are undefined.
lR
Greek letters (in Roman type) are used in inorganic nomenclature as follows:
to show absolute configuration, or as a structural descriptor to designate deltahedra
(see Section IR-9.3.4);
δ
to denote the absolute configuration of chelate rings (see Section IR-9.3.4); in
solid state chemistry to indicate small variations of composition (see Section IR11.3.2); to designate cumulative double bonds in ring or ring systems (see Section
P-25.7.2 of Ref 1);
η
to designate the hapticity of a ligand (see Sections IR-9.2.4.3 and IR-10.2.5.1);
κ
as a ligating atom designator in the kappa convention (see Sections IR-9.2.4.2 and
IR-10.2.3.3);
Pr
ov
isi
o
na
∆
to show absolute configuration (see Section IR-9.3.4);
λ
to indicate non-standard bonding number in the lambda convention (see Section
IR-6.2.1 and Section P-14.1 of Ref.1); to denote the absolute configuration of
chelate rings (see Section IR-9.3.4);
µ
to designate a bridging ligand (see Sections IR-9.2.5.2 and IR-10.2.3.1).
IU
PA
C
Λ
IR-2.11
Page 23 of 33
ASTERISKS
DRAFT 2 April 2004
24
tio
n
s
The asterisk (*) is used in formulae as a right superscript to the symbol of an element, in
the following ways:
(a) To highlight a chiral centre.
da
Example:
1.
H
H2C
C*
H
om
me
n
C
CH3
CHMe2
ec
This usage has been extended to label a chiral ligand or a chiral centre in coordination
chemistry.
Example:
Me
C
*
S
*
C
V
S
ov
isi
o
Ph
na
H
lR
2.
Pr
(b) To designate excited molecular or nuclear states.
PA
C
Example:
3.
IU
IR-2.12
NO*
PRIMES
(a) Primes ('), double primes (''), triple primes ('''), etc. may be used in the names and
formulae of coordination compounds in the following ways:
(i) within ligand names, in order to differentiate between sites of substitution;
DRAFT 2 April 2004
Page 24 of 33
25
s
(ii) when specifying donor atoms (IR-9.2.4.2), in order to differentiate between
donor atoms;
da
[Rh3Cl(µ-Cl)(CO)3{µ3-Ph2PCH2P(Ph)CH2PPh2}2]+
om
me
n
Example:
1.
tio
n
(iii) when specifying stereochemistry using configuration indices (IR-9.3.5.3), in
order to differentiate between donor atoms of the same priority, depending on
whether they are located within the same ligand or portion of the ligand.
+
Ph
Ph2P
P
CO
OC
Rh
Rh
Ph2P
Cl
Rh
OC
P
ec
Cl
PPh2
PPh2
lR
Ph
tricarbonyl-1κC,2κC,3κC-µ-chlorido-1:2κ2Cl-chlorido-3κCl-bis{µ3-
na
bis[(diphenylphosphanyl)-1κP':3κP''-methyl]phenylphosphane-2κP}trirhodium(1+)
MULTIPLICATIVE PREFIXES
PA
C
IR-2.13
x
•
′ x
LiLi,1-2
x MgLi,xVLi,x ClCl
Pr
Example:
2.
ov
isi
o
(b) Primes, double primes, triple primes, etc. are also used as right superscripts in the
Kröger-Vink notation (see Section IR-11.4) where they indicate a site which has one, two,
three, etc. units of negative effective charge.
IU
The number of identical chemical entities in a name is expressed by a numerical prefix
(see Table IV).
Page 25 of 33
In the case of simple entities such as monoatomic ligands the multiplicative prefixes di,
tri, tetra, penta, etc., are used.
DRAFT 2 April 2004
26
diiron trioxide
_
[PtCl4]2
[Fe(CCPh)2(CO)4]
TlI3
Ca3(PO4)2
Pt(PPh3)4
tetrachloridoplatinate(2_)
tetracarbonylbis(phenylethynyl)iron
thallium(III) tris(iodide)
tricalcium bis(phosphate)
tetrakis(triphenylphosphane)platinum(0)
da
2.
3.
4.
5.
6.
Fe2O3
om
me
n
Examples:
1.
tio
n
s
The multiplying prefixes bis, tris, tetrakis, pentakis, etc. are used with organic ligands
(particularly if substituted) or to avoid ambiguity (even with simple ligands, see Examples
4 and 5 below). The modified entity is placed within parentheses.
LOCANTS
IR-2.14.1
Introduction
lR
IR-2.14
ec
Composite numeral prefixes are built up by citing units first, then tens, hundreds and so
on, e.g. 35 is written pentatriaconta (or pentatriacontakis).
Arabic numerals
isi
o
IR-2.14.2
na
Locants are used to indicate the position of a substituent or structural feature on or within
a parent molecule. These can be arabic numerals or lower case letters.
ov
Arabic numerals are used as locants in the following ways.
Pr
(a) For numbering skeletal atoms in parent hydrides, to indicate: the placement of
hydrogen atoms when there are non-standard bonding numbers; unsaturation; the positions
of bridging hydrogen atoms in a borane structure.
IU
PA
C
Examples:
1.
1 23
4
H 5SSSH4SH
1λ6, 3λ6-tetrasulfane (not 2λ6, 4λ6)
12
3
45
2.
H 2NN=NHNNH2
3.
2,3:2,5:3,4:4,5-tetra-µH-nido-pentaborane(9)
pentaaz-2-ene
DRAFT 2 April 2004
Page 26 of 33
27
s
(b) In replacement nomenclature.
11 10 9
8
7 6
5
4
3
tio
n
Example:
4.
21
(c) In additive nomenclature.
Example:
5.
1
2
3
4
5
SiH3GeH2SiH2SiH2SiH3
om
me
n
7,10-dioxa-2-thia-4-silaundecane
da
CH3OCH2 CH2 OCH2 CH2 SiH2 CH2 SCH3
1,1,1,2,2,3,3,4,4,5,5,5-dodecahydrido-2-germy-1,3,4,5-tetrasily-[5]catena
Example:
6.
1
4
HSb
2
na
O
lR
ec
(d) In the Hantzsch-Widman nomenclature, to indicate positions of skeletal atoms.
SbH
O
isi
o
3
ov
1,3,2,4-dioxadistibetane
Pr
(e) In the Hantzsch-Widman nomenclature, to denote indicated hydrogen.
IU
PA
C
Example:
7.
3
HSi
2
H
Ge
SiH2
1
1H-1,2,3-germadisilirene
(f) In substitutive nomenclature, to specify the positions of substituent groups.
Example:
Page 27 of 33
DRAFT 2 April 2004
28
8.
1
2
3
4
5
HOSiH2SiH2SiH2SiHClSiH2Cl
tio
n
s
4,5-dichloropentasilane-1-ol
(g) In substitutive nomenclature, to illustrate a subtractive operation.
Example:
9.
da
•NHNH• and −NHNH−
hydrazine-1,2-diyl
Example:
9.
H
Si
H
Si
HSi
Si
8
Si
7
1
2
3
6
4
SiH
SiH
ec
HSi 9 10
om
me
n
(h) In von Baeyer names to indicate the topology of a polycyclic ring system.
5
Si
H
lR
Si
H
na
bicyclo[4.4.0]decasila-1,3,5,7,9-pentaene
ov
Example:
10.
isi
o
(i) In polynuclear coordination compounds, for numbering the central atoms (see Section
IR-9.2.5).
1 2
nonacarbonyl-1κ5C,2κ4C-rheniumcobalt(Re—Co)
Pr
[(OC)5ReCo(CO)4]
PA
C
(j) To indicate stereochemistry at particular atoms in structures where arabic numerals
have been used for numbering those atoms.
IU
Example:
11.
IR-2.14.3
1
2
3
4
5
ClSiH2SiHClSiHClSiH2SiH3
(2R,3S)-1,2,3-trichloropentasilane
Lower case letters
DRAFT 2 April 2004
Page 28 of 33
29
PRIORITIES
IR-2.15.1
Introduction
da
IR-2.15
tio
n
s
Lower case letters are used in polyoxometallate nomenclature to designate the vertices of
the coordination polyhedra around the central atoms. They are attached to the number of
the central atom to which a particular vertex refers. A detailed treatment is given in
Chapter II-1 of Ref. 2.
om
me
n
In chemical nomenclature the terms priority, seniority and precedence convey the notion
of rank or order among a number of possibilities and are ubiquitous concepts of
fundamental importance. Chemical nomenclature deals with elements and their
combinations among themselves, either individually or as groups (element with element;
group with group). The groups of atoms can be ions, ligands in coordination compounds,
or substituents in hydrides.
IR-2.15.2
na
lR
ec
Whereas writing the symbol or the name of an element is straightforward, a choice of
which element to write first in the formula and in the name has to be made as soon as one
element is associated with a second to form, for example, a binary compound. The order
of citation is based upon the methods outlined below.
Electronegativity criterion
Pr
ov
isi
o
In formulae and compositional names of binary compounds of non-metallic elements, the
constituent which appears earlier in the sequence below is cited first. This is an
approximate electronegativity sequence, though it departs in detail from the usually
accepted electronegativity sequence, for example in the relative positions of C and H (see
Table VI).
Rn, Xe, Kr, Ar, Ne, He, B, Si, C, Sb, As, P, N, H, Te, Se, S, At, I, Br, Cl, O, F.
PA
C
Example:
1.
IU
IR-2.15.3
S2Cl2
Alphabetical order
Alphabetical order is used in names as follows.
(a) In coordination compounds, to define the order of citation of ligands. This order
follows the alphabetical listing of the names of the ligands. The alphabetical citation of
Page 29 of 33
DRAFT 2 April 2004
30
s
ligands is maintained regardless of the number of each ligand, or whether the compounds
are mono- or polynuclear.
1.
tio
n
Example:
potassium (disulfido)sulfidoaurate(1_)
K[AuS(S2)]
om
me
n
da
The term 'coordination compound' is extended here to include compounds where two or
more different atoms or groups are attached to a single central atom, regardless of whether
this central atom is a metal or not.
(b) In salts, the cations and the anions are each arranged alphabetically with cations
preceding anions. However, deviation is allowed when structural relationships between
different compounds are being emphasized.
magnesium potassium fluoride
ZnI(OH)
NaNbO3
zinc hydroxide iodide
niobium sodium trioxide (perovskite type)
lR
3.
4.
KMgF3
ec
Examples:
2.
Alphabetical order is used in formulae as follows.
ov
[CrCl2(NH3)2(OH2)2]
Pr
Example:
5.
isi
o
na
(a) In coordination compounds, ligands are cited in alphabetical order of the first symbols
of the formulae or abbreviations that are used. Where possible, the donor atom should be
placed nearest the central atom to which it is coordinated. Some deviation is allowed if it
is desired to convey specific structural information. (See Section IR-9.2.5.1)
PA
C
(b) In salts and double salts, to establish the sequential orders in cations and anions,
respectively.
IU
Examples:
6.
7.
IR-2.15.4
BiClO
KNa4Cl(SO4)2
Element priority sequence
This sequence is based on the Periodic Table and is shown in Table VI. The element
columns (1 to 18) are connected by arrows leading in a direction starting from the less
DRAFT 2 April 2004
Page 30 of 33
31
s
metallic elements and moving towards the more metallic elements. This order had its
origin in electronegativity considerations. It is followed in the situations cited below.
tio
n
(a) In polynuclear coordination compounds:
To order the central atoms in names (IR-9.2.5.1). The first central atom
encountered when following the arrow is listed last.
(ii)
To number the central atoms for use in conjunction with the kappa
convention (IR-9.2.5.1). The last central atom reached in the direction of
the arrow is given the lowest number. This number will represent the
position of the central atom in the list of central atoms that forms part of
the name.
om
me
n
da
(i)
Other priority sequences
IR-2.15.5.1
Organic priority orders
na
IR-2.15.5
lR
ec
(b) Where there is a choice of parent hydride among those listed in Table IR-6.1 (or
corresponding hydrides with non-standard bonding numbers, cf. Section IR-6.2.2.2), the
name is based on the parent hydride of the element occurring first in the sequence: N, P,
As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, Tl, O, S, Se, Te, C, F, Cl, Br, I.
Priority of ligand types
Pr
IR-2.15.5.2
ov
isi
o
In substitutive nomenclature, an order for the choice of principal functional group is
defined (see Section P-41 of Ref.1). In coordination compounds, the organic ligands that
would normally be named by substitutive nomenclature retain their substitutive names
with the chosen principal group expressed as a suffix (modified to designate a ligand).
IU
PA
C
In formulae of coordination compounds, ligands are cited in alphabetical order. Bridging
ligands are cited after terminal ligands, and in increasing order of bridging multiplicity.
(See also Sections IR-9.2.3 and IR-9.2.5).
Page 31 of 33
In names of coordination compounds, the ligands precede the metal and are cited in
alphabetical order of the ligand name. For each ligand, bridging ligands are cited before
terminal ligands, e.g. di-µ-chlorido-tetrachlorido, and in decreasing order of bridging
multiplicity, e.g. µ3-oxido-di-µ-trioxido.... (See also Sections IR-9.2.2 and IR-9.2.5).
DRAFT 2 April 2004
32
Priorities in salt formulae and names
tio
n
IR-2.15.5.3
s
Thus, for both formulae and names the terminal ligands are closer to the metal, with the
multiplicity of the bridging ligands increasing further away from the metal.
Isotope labelling and modification
om
me
n
IR-2.15.5.4
da
In formulae and names of salts, double salts, and coordination compounds, cations precede
anions.
In isotopically modified compounds, a priority principle governs the order of citation of
nuclide symbols. (See Section II-2.2.5 of Ref. 2).
IR-2.15.5.5
Stereochemical priorities
Priority sequences of punctuation marks
na
IR-2.15.5.6
lR
ec
In the stereochemical nomenclature of coordination compounds, the procedure for
assigning priority numbers to the ligating atoms of a mononuclear coordination system is
based upon the standard sequence rules developed for chiral carbon compounds (the Cahn,
Ingold, Prelog or CIP rules5, see Section IR-9.3.3.2).
comma < colon < semicolon.
Pr
ov
isi
o
In the names of coordination compounds and boron compounds, the punctuation marks
used to separate the symbols of atoms from the numerical locants, the locants indicating
bridging atoms, and the various other sets of locants which may be present, are arranged
in the following hierarchy:
PA
C
The colon is only used for bridging ligands, so that the more restricted general hierarchy is
simply comma < semicolon. The sequence when bridging ligands are being specified is
comma < colon. (See Example 2 in Section IR-2.5.2, and Section IR-9.2.5.5).
IU
IR-2.16
IR-2.17
AFFIXES (Prefixes, suffixes and infixes)
Any name more complex than a simple element name has a structure, i.e. a root with a
prefix or a suffix. The suffix is a terminal vowel or a combination of letters. Some
common affixes are listed in Tables III-V.
FINAL REMARKS
DRAFT 2 April 2004
Page 32 of 33
33
om
me
n
REFERENCES
New Blue Book title needed to replace: Nomenclature of Organic Chemistry,
Pergamon Press, Oxford, 1979; A Guide to IUPAC Nomenclature of Organic
Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford,
1993; Pure Appl. Chem., 71, 1327 (1999).
2.
Nomenclature of Inorganic Chemistry II, Recommendations 2000, Royal Society of
Chemistry, 2001.
3.
Compendium of Macromolecular Nomenclature, Blackwell Scientific Publications,
Oxford, 1991.
4.
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific
Publications, Oxford, 1990.
5.
6.
J. B. Casey, W.J. Evans and W.H. Powell, Inorg. Chem., 20, 1333 (1981).
R.S. Cahn, C. Ingold and V. Prelog, Angew. Chem., Int. Ed. Engl., 5, 385 (1966); V.
Prelog and G. Helmchen, Angew. Chem., Int. Ed. Engl., 21, 567 (1982).
na
lR
ec
1.
IU
PA
C
Pr
ov
isi
o
IR-2.18
da
tio
n
s
In this Chapter, the various uses of letters, numerals and symbols in names and formulae
have been gathered under common headings to provide an easy check to ensure that the
constructed name or formula is in accord with agreed practice. However, this Chapter is
not sufficient to make clear all the rules needed to build a name or a formula, and the
reader is therefore advised to consult other appropriate Chapters for the more detailed
treatment.
Page 33 of 33
DRAFT 2 April 2004
1
Elements and Groups of Elements
(March 04)
s
IR-3
tio
n
CONTENTS
om
me
n
da
IR-3.1 Names and symbols of atoms
IR-3.1.1 Systematic nomenclature and symbols for new elements
IR-3.2 Indication of mass, charge and atomic number using indexes (subscripts and
superscripts)
IR-3.3 Isotopes
IR-3.3.1 Isotopes of an element
IR-3.3.2 Isotopes of hydrogen
IR-3.4 Elements (or elementary substances)
ov
isi
o
na
lR
ec
IR-3.4.1 Name of an element of infinite or indefinite molecular formula or
structure
IR-3.4.2 Name of allotropes of definite molecular formula
IR-3.5 Allotropic modifications
IR-3.5.1 Allotropes
IR-3.5.2 Allotropic modifications constituted of discrete molecules
IR-3.5.3 Crystalline allotropic modifications of an element
IR-3.5.4 Solid amorphous modifications and commonly recognized allotropes of
indefinite structure
IR-3.6 Groups of elements
IR-3.6.1 Groups of elements in the Periodic Table and their subdivisions
IR-3.6.2 Collective names of groups of like elements
IR-3.7 References
NAMES AND SYMBOLS OF ATOMS
Pr
IR-3.1
IU
PA
C
The origins of the names of some chemical elements, for example antimony, are lost in
antiquity. Other elements recognised (or discovered) during the past three centuries were
named according to various associations of origin, physical or chemical properties, etc.,
and more recently to commemorate the names of eminent scientists.
Page 1 of 9
In the past, some elements were given two names because two groups claimed to have
discovered them. To avoid such confusion it was decided in 1947 that after the existence
of a new element had been proved beyond reasonable doubt, discoverers had the right to
suggest a name to IUPAC, but that only the Commission on Nomenclature of Inorganic
Chemistry (CNIC) could make a recommendation to the IUPAC Council to make the final
DRAFT 2 April 2004
2
s
decision. Names for elements up to and including element 103 do not, therefore, carry any
implication regarding priority of discovery.
om
me
n
da
tio
n
Under the present procedure,1 claims of the discovery of a new element are first
investigated by a joint IUPAC-IUPAP (International Union of Pure and Applied Physics)
committee which then assigns priority. The discoverers are then invited to suggest a name
to the Division of Inorganic Chemistry before a formal recommendation to the IUPAC
Council is made. It is emphasised that the IUPAC-approved name for any new element
carries no implication regarding priority of discovery.
The IUPAC-approved names of the atoms of atomic numbers 1-110 for use in the English
language are listed in alphabetical order in Table I*; it is desirable that the names used in
other languages differ from these as little as possible. Names which are not used in
English (but which provide the basis of the atomic symbol), which have entered into
chemical nomenclature, or which are IUPAC-approved alternatives are noted in Table I.
Systematic nomenclature and symbols for new elements
na
IR-3.1.1
lR
ec
For use in chemical formulae, each atom is represented by a unique symbol in upright type
as shown in Table I. In addition, the symbols D and T may be used for the hydrogen
isotopes of mass numbers two and three respectively (see Section IR-3.3.2).
ov
isi
o
Newly discovered elements may be referred to in the scientific literature but until they
have received permanent names and symbols from IUPAC, temporary designators are
required. Such elements may be referred to by their atomic numbers, as in 'element 120'
for example, but IUPAC has approved a systematic nomenclature and series of three-letter
symbols (see Table II).2
Pr
The name is derived directly from the atomic number of the element using the following
numerical roots:
IU
PA
C
0 = nil
1 = un
2 = bi
3 = tri
4 = quad
5 = pent
6 = hex
7 = sept
8 = oct
9 = enn
The roots are put together in the order of the digits which make up the atomic number and
terminated by 'ium' to spell out the name. The final 'n' of 'enn' is elided when it occurs
before 'nil', and the final 'i' of 'bi' and of 'tri' when it occurs before 'ium'.
*
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
Page 2 of 9
3
tio
n
s
The symbol for the element is composed of the initial letters of the numerical roots which
make up the name.
Example:
da
113 = ununtrium, symbol Uut
INDICATION OF MASS, CHARGE AND ATOMIC NUMBER USING INDEXES
(SUBSCRIPTS AND SUPERSCRIPTS)
om
me
n
IR-3.2
ec
The mass, charge and atomic number of a nuclide are indicated by means of three indexes
(subscripts and superscripts) placed around the symbol. The positions are occupied as
follows:
left upper index
mass number
left lower index
atomic number
right upper index
charge
lR
_
_
A charge placed on an atom of symbol A is indicated as An+ or An , not as A+n or A n.
na
The right lower position of an atomic symbol is reserved for an index (subscript)
indicating the number of such atoms in a formula. For example, S8 is the formula of a
32 2+
16
S
represents a doubly ionized sulfur atom of atomic number 16 and
ov
Example:
1.
isi
o
molecule containing eight sulfur atoms (see Section IR-3.4). For formalisms when
oxidation states or charges are also shown, see Section IR-4.6.1.
Pr
mass number 32.
IU
PA
C
4
1
29
The nuclear reaction between 26
12 Mg and 2 He nuclei to yield 13Al and 1H nuclei is
written as follows3:
IR-3.3
Page 3 of 9
26Mg(α,p)29Al
For the nomenclature of isotopically modified compounds and the use of atomic symbols
to indicate isotopic modification in chemical formulae see Section IR-4.5 and Chapter II-2
of Ref. 4.
ISOTOPES
DRAFT 2 April 2004
4
IR-3.3.1
Isotopes of an element
Isotopes of hydrogen
da
IR-3.3.2
tio
n
s
The isotopes of an element all bear the same name (but see Section IR-3.3.2) and are
designated by mass numbers (see Section IR-3.2). For example, the atom of atomic
number 8 and mass number 18 is named oxygen-18 and has the symbol 18O.
om
me
n
Hydrogen is an exception to the rule in Section IR-3.3.1 in that the three isotopes 1H, 2H,
and 3H can have the alternative names protium, deuterium, and tritium, respectively. The
symbols D and T may be used for deuterium and tritium but 2H and 3H are preferred
because D and T can disturb the alphabetical ordering in formulae (see Chapter IR-4.5).
Furthermore, the combination of a muon and an electron behaves like a light isotope of
hydrogen and is named muonium, symbol Mu.5
na
lR
ec
These names give rise to the names proton, deuteron, triton, and muon for the cations 1H+,
2H+, 3H+, and Mu+, respectively. Because the name proton is often used in contradictory
senses, i.e. for isotopically pure 1H+ ions on the one hand, and for the naturally occurring
undifferentiated isotope mixture on the other, it is recommended that the undifferentiated
mixture be designated generally by the name hydron, derived from hydrogen.
ELEMENTS (or elementary substances)
IR-3.4.1
Name of an element of infinite or indefinite molecular formula or structure
isi
o
IR-3.4
Names of allotropes of definite molecular formula
Pr
IR-3.4.2
ov
A sample of an element that has an undefined formula, or is a mixture of allotropes (see
Section IR-3.5), bears the same name as the atom.
IU
PA
C
Allotropes of definite molecular formula are named by adding the appropriate numerical
prefix (Table IV) (mono, di, tri, tetra, penta, etc.) to designate the number of atoms in the
molecule. The prefix 'mono' is not used except when the element does not normally exist
in a monoatomic state.
Examples:
1.
2.
3.
H, monohydrogen
N, mononitrogen
N2, dinitrogen
5.
6.
7.
DRAFT 2 April 2004
O3, trioxygen (ozone)
P4, tetraphosphorus
S8, octasulfur
Page 4 of 9
5
4.
Ar, argon
Example:
8.
tio
n
s
The prefic 'poly' may be used if the number of atoms in the molecule is large and
unknown.
ALLOTROPIC MODIFICATIONS
IR-3.5.1
Allotropes
om
me
n
IR-3.5
da
Sn, polysulfur
Allotropic modifications constituted of discrete molecules
ov
IR-3.5.2
isi
o
na
lR
ec
Allotropic modifications of an element bear the name of the atom from which they are
derived, together with a descriptor to specify the modification. Common descriptors are
Greek letters (α, β, γ, etc.), colours and, where appropriate, mineral names (e.g. graphite
and diamond for the well known forms of carbon). Such names should be regarded as
provisional, to be used only until structures have been established, after which a rational
system based on molecular formula (see Section IR-3.5.2) or crystal structure (see Section
IR-3.5.3) is recommended. The well-established common names or descriptors are
allowed alternatives for common structurally defined allotropes of carbon, phosphorus,
sulfur, tin and iron (see Examples in Chapter IR-11, and in Section IR-3.5.3), except when
they can be treated under Section IR-3.5.2. Common names will also continue to be used
for amorphous modifications of an element and for those which are mixtures of closely
related structures (such as graphite) in their commonly occurring forms or have an illdefined disordered structure (such as red phosphorus) (see Section IR-3.5.4).
PA
C
Pr
Systematic names are based on the number of atoms in the molecule, indicated by a
numerical prefix (see Section IR-3.4.2). If the number is large and unknown, as in long
chains or large rings, the prefix 'poly' may be used. Where necessary, appropriate prefixes
(Table V) may be used to indicate structure. When it is desired to specify a particular
polymorphous form of a molecular element with a defined structure (such as S8 in α- or β-
IU
sulfur) the method of Section IR-3.5.3 should be used.
Page 5 of 9
Examples:
Symbol
1.
O2
2.
O3
Systematic name
dioxygen
trioxygen
DRAFT 2 April 2004
Common name
oxygen
ozone
6
4.
5.
6.
white phosphorus
hexasulfur
octasulfur
polysulfur
(or yellow phosphorus)
α-sulfur, β-sulfur
µ-sulfur (or plastic sulfur)
Crystalline allotropic modifications of an element
da
IR-3.5.3
S6
S8
Sn
tetraphosphorus
s
P4
tio
n
3.
om
me
n
Crystalline allotropic modifications are polymorphs of the elements. Each can be named
by adding the Pearson symbol6 in parentheses after the name of the atom. This symbol
defines the structure of the allotrope in terms of its Bravais lattice (crystal class and type
of unit cell) and number of atoms in its unit cell (Table IR-3.1 and Chapter IR-11). Thus,
iron(cF4) is the allotropic modification of iron (γ-iron) with a cubic (c), all-face-centred
(F) lattice containing four atoms of iron in the unit cell. The trivial nomenclature of
allotropes is discouraged.
ec
Table IR.3.1 Pearson symbols used for the fourteen Bravais lattices
lR
Lattice symbol a
System
Triclinic
na
Monoclinic
P
aP
mP
Sb
mS
P
oP
S
F
I
oS
oF
oI
P
tP
I
tI
Hexagonal
(and trigonal P)
P
hP
Rhombohedral
R
hR
Cubic
P
cP
F
I
cF
cI
isi
o
P
Pr
ov
Orthorhombic
PA
C
Tetragonal
IU
Pearson symbol
DRAFT 2 April 2004
Page 6 of 9
7
s
a P, S, F, I, and R are primitive, one-face-centred, all-face-centred, body-centred and
rhombohedral lattices, respectively. b Second setting, y-axis unique.
tio
n
In a few cases, the Pearson symbol fails to differentiate between two crystalline allotropes
of the element. In such an event the space group is added to the parentheses. Thus, for
example, the two forms of Se, α-selenium and β-selenium, both Sen, are distinguished by
da
the symbols (mP32, P21/n) and (mP32, P21/a), respectively. Alternatively, a notation
involving compound type may be useful (see Chapter IR-11).
carbon(hR6)
iron(cI2)
iron(cF4)
tin(cF8)
tin(tI4)
manganese(cI58)
manganese(cP20)
manganese(cF4)
manganese(cI2)
sulfur(hR18)
sulfur(oP80)
graphite (less common form)
α-iron
γ−iron
α- or grey tin
β- or white tin
α-manganese
β-manganese
γ-manganese
δ-manganese
-
lR
ec
Common name
black phosphorus
diamond
graphite (common form)
na
Cn
Cn
Fen
Fen
Snn
Snn
Mnn
Mnn
Mnn
Mnn
S6
S20
Systematic name
phosphorus(oC8)
carbon(cF8)
carbon(hP4)
Solid amorphous modifications and commonly recognized allotropes of
ov
IR-3.5.4
Symbol
Pn
Cn
isi
o
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
om
me
n
Examples:
Pr
indefinite structure
IU
PA
C
Solid amorphous modifications and commonly recognized allotropes of indefinite
structure are distinguished by customary descriptors such as a Greek letter, names based
on physical properties, or mineral names (see Examples in Section IR-3.5.3).
Examples:
1.
2.
3.
Cn
Cn
vitreous carbon
graphitic carbon (carbon in the form of graphite, irrespective of
Pn
structural defects)
red phosphorus [a disordered structure containing parts of
phosphorus(oC8) and parts of tetraphosphorus]
Page 7 of 9
DRAFT 2 April 2004
8
4.
Asn
amorphous arsenic
GROUPS OF ELEMENTS
IR-3.6.1
Groups of elements in the Periodic Table and their subdivisions
tio
n
s
IR-3.6
Collective names of groups of like elements
ec
IR-3.6.2
om
me
n
da
The groups of elements in the Periodic Table are numbered from 1 to 18. The elements
(except hydrogen) of groups 1, 2, 13, 14, 15, 16, 17 and 18 are designated as Main Group
elements and, except in group 18, the first two elements of each Main Group are termed
Typical Elements. The elements of groups 3-11 are Transition Elements. Optionally the
letters s, p, d and f may be used to distinguish different blocks of elements. If appropriate
for a particular purpose, the various groups may be named from the first element in each,
for example elements of the boron group (B, Al, Ga, In, Tl), elements of the titanium
group (Ti, Zr, Hf, Rf), etc.
isi
o
na
lR
The following collective names for groups of atoms are IUPAC-approved: alkali metals
(Li, Na, K, Rb, Cs, Fr), alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), pnictogens (N, P,
As, Sb, Bi), chalcogens (O, S, Se, Te, Po), halogens (F, Cl, Br, I, At), noble gases (He, Ne,
Ar, Kr, Xe, Rn), lanthanoids (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb,
Lu), rare earth metals (Sc, Y and the lanthanoids), and actinoids (Ac, Th, Pa, U, Np, Pu,
Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr).
ov
The generic terms pnictide, chalcogenide, and halogenide (or halide) are used in naming
compounds of the pnictogens, chalcogens, and halogens.
IU
PA
C
Pr
Although lanthanoid means 'like lanthanum' and so should not include lanthanum,
lanthanum has become included by common usage. Similarly, actinoid. The ending 'ide'
normally indicates a negative ion, and therefore lanthanoid and actinoid are preferred to
lanthanide and actinide. However, lanthanide and actinide are still allowed owing to wide
current use.
A transition element is an element whose atom has an incomplete d-sub-shell, or which
gives rise to a cation or cations with an incomplete d-sub-shell. The First Transition Series
of elements is Sc, Ti, V, Cr, Mn, Fe, Co, Ni and Cu. The Second and Third Transition
Series are similarly derived: these include the lanthanoids and actinoids respectively. The
latter are designated inner (or f) transition elements of their respective Periods in the
Periodic Table.
DRAFT 2 April 2004
Page 8 of 9
9
REFERENCES
4.
5.
s
IU
PA
C
Pr
ov
isi
o
na
lR
ec
6.
tio
n
3.
Naming of New Elements, Pure Appl. Chem., 74, 787 (2002).
Recommendations for the Naming of Elements of Atomic Numbers Greater Than
100, Pure Appl. Chem., 51, 381 (1979).
Quantities, Units and Symbols in Physical Chemistry, Second Edn, Blackwell
Scientific Publications, Oxford, 1993. (Update not yet published).
Nomenclature of Inorganic Chemistry II, Recommendations 2000, Royal Society of
Chemistry, 2001.
Names for Muonium and Hydrogen Atoms and Their Ions, Pure Appl. Chem., 73,
377 (2001).
W.B. Pearson, A Handbook of Lattice Spacings and Structures of Metals and Alloys,
Vol. 2, Pergamon Press, Oxford, 1967, pp. 1,2. For tabulated lattice parameters and
data on elemental metals and semi-metals, see pp. 79-91. See also, P. Villars and
L.D. Calvert, Pearson's Handbook of Crystallographic Data for Intermetallic
Phases, Vols. 1-3, American Society for Metals, Metals Park, Ohio, USA, 1985.
da
1.
2.
om
me
n
IR-3.8
Page 9 of 9
DRAFT 2 April 2004
1
IR-4
Formulae (Draft March 2004)
tio
n
s
CONTENTS
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
IR-4.1 Introduction
IR-4.2 Definitions of types of formula
IR-4.2.1 Empirical formulae
IR-4.2.2 Molecular formulae
IR-4.2.3 Structural formulae and the use of enclosing marks in formulae
IR-4.2.4 Formulae of addition compounds
IR-4.2.5 Solid state structural information
IR-4.3 Indication of ionic charge
IR-4.4 Sequence of citation of symbols in formulae
IR-4.4.1 Introduction
IR-4.4.2 Ordering principles
IR-4.4.2.1 Electronegativity
IR-4.4.2.2 Alphabetical order
IR-4.4.3 Formulae for specific classes of compounds
IR-4.4.3.1 Binary compounds
IR-4.4.3.2 (Formal) treatment as coordination compounds
IR-4.4.3.3 Chain compounds
IR-4.4.3.4 Generalised salt formulae
IR-4.4.3.5 Addition compounds, multiple salts, and solvates
IR-4.4.4 Use of ligand abbreviations
IR-4.5 Isotopically modified compounds
IR-4.5.1 General formalism
IR-4.5.2 Isotopically substituted compounds
IR-4.5.3 Isotopically labelled compounds
IR-4.5.3.1 Types of labelling
IR-4.5.3.2 Specifically labelled compounds
IR-4.5.3.3 Selectively labelled compounds
IR-4.6 Optional modifiers of formulae
IR-4.6.1 Indication of oxidation state
IR-4.6.2 Formulae of radicals
IR-4.6.3 Formulae of optically active compounds
IR-4.6.4 Indication of excited states
IR-4.6.5 Structural descriptors
IR-4.7 References
Page 1 of 17
DRAFT 2 April 2004
2
IR-4.1
INTRODUCTION
IR-4.2.1
Empirical formulae
da
DEFINITIONS OF TYPES OF FORMULA
om
me
n
IR-4.2
tio
n
s
Formulae (empirical, molecular, and structural formulae as described below) provide a simple
and clear method of designating compounds. They are of particular importance in chemical
equations and in descriptions of chemical procedures. In order to avoid ambiguity and for
many other purposes, e.g. in databases, indexing, etc., standardisation is recommended.
Molecular formulae
isi
o
IR-4.2.2
BrClH3N2NaO2Pt
C10H10ClFe
na
Examples:
1.
2.
lR
ec
The empirical formula of a compound is formed by juxtaposition of the atomic symbols with
appropriate (integer) subscripts to give the simplest possible formula expressing the
composition. For the order of citation of symbols in formulae, see Section IR-4.4, but, in the
absence of any other ordering criterion (for example, if little structural information is
available), the alphabetical order of atomic symbols should be used in an empirical formula,
except that in carbon-containing compounds, C and H are usually cited first and second,
respectively.1
ov
For compounds consisting of discrete molecules, the molecular formula, as opposed to the
empirical formula, may be used to indicate the actual composition of the molecules. For the
order of citation of symbols in molecular formulae, see Section IR-4.4.
PA
C
Pr
The choice of formula depends on the chemical context. In some cases, the empirical formula
may also correspond to a molecular composition, in which case the only possible difference
between the two formulae is the ordering of the atomic symbols. If it is not desirable or
possible to specify the composition, e.g. in the case of polymers, a letter subscript such as n
may be used.
IU
Examples:
1.
2.
3.
3.
Molecular formula
S8
Sn
SF6
S2Cl2
Empirical formula
S
S
F 6S
ClS
DRAFT 2 April 2004
Page 2 of 17
3
5.
6.
Hg2Cl2
N 2O 4
H 2O 3P
ClHg
NO2
s
H 4P 2O 6
tio
n
IR-4.2.3
4.
Structural formulae and the use of enclosing marks in formulae
Examples:
1.
2.
3.
om
me
n
da
A structural formula gives partial or complete information about the way in which the atoms
in a molecule are connected and arranged in space. In simple cases, a line formula that is just
a sequence of atomic symbols gives structural information provided the reader knows that the
formula represents the order of the atoms in the linear structure.
HOCN
HNCO
HOOH
(empirical formula CHNO)
(empirical formula also CHNO)
(empirical formula HO)
lR
ec
As soon as the compound has even a slightly more complex structure, it becomes necessary
to use enclosing marks in line formulae to separate subgroups of atoms. Different enclosing
marks must be used for repeating units and sidechains in order to avoid ambiguity.
(i)
na
The basic rules for applying enclosing marks in structural formula are as follows:
Repeating units in chain compounds are enclosed in square brackets.
ov
isi
o
(ii)
Side groups to a main chain and groups (ligands) attached to a central atom
are enclosed in parentheses (except single atoms when there is no ambiguity
regarding their attachment in the structure, e.g. hydrogen in hydrides with a chain
structure).
PA
C
Pr
(iii)
A formula or part of a formula which represents a molecular entity may be
placed in enclosing marks. If an entire formula is enclosed, square brackets must be
used, except if rule (v) applies.
IU
(iv)
A part of a formula which is to be multiplied by a subscript may also be
enclosed in parentheses or braces, except in the case of repeating units in chain
compounds, cf. rule (i).
Page 3 of 17
(v)
In the case of polymers, if the bonds between repeating units are to be shown,
the repeating unit is enclosed in strike-through parentheses, with the dash
superimposed on the parentheses representing the bond. (If this is typographically
inconvenient, dashes can be placed before and after the parentheses).
DRAFT 2 April 2004
4
Inside square brackets, enclosing marks are nested as follows:
( ), {( )}, ({( )}), {({( )})}, etc.
s
(vi)
tio
n
(vii)
Atoms or groups of atoms which are represented together with a prefixed
symbol, e.g. a structural modifier such as 'µ', are placed within enclosing marks, using
the nesting order ( ), {( )}, ({( )}), {({( )})}, etc.
om
me
n
da
The use of enclosing marks for the specification of isotopic substitution is described in
Section IR-4.5.
Compared to line formulae, displayed formulae (Example 13 below) give more (or full)
information about the structure.
isi
o
na
lR
SiH3[SiH2]8SiH3
SiH3[SiH2]6SiH(SiH3)SiH3
Ca3(PO4)2
[Co(NH3)6]2(SO4)3
[{Rh(µ-Cl)(CO)2}2]
K[Os(N)3]
(S)n
(HBO2)n, or (B(OH)O)n
([PdCl2])n, or
ov
Examples:
4.
5.
6.
7.
8.
9.
10.
11.
12.
ec
(The rules needed for ordering the symbols in some of the example formulae below are given
in Section IR-4.4.3.)
Cl
Pd
Pr
PA
C
[rule (i)]
[rules (i) and (ii)]
[rule (iv)]
[rules (iii), (iv), (vi)]
[rules (iii), (vi), (vii)]
[rules (ii), (iii)]
[rule (v)]
[rules (ii) and (v)]
Cl
n
IU
13.
14.
15.
Cl
PPh3
Ni
Cl
PPh3
NaCl
[NaCl]
DRAFT 2 April 2004
Page 4 of 17
5
tio
n
s
The first formula in each of Examples 11 and 12 may be considered to be molecular
formulae (Section IR-4.2.2) with no implications about the structure of the polymers in
question.
Formulae of addition compounds
om
me
n
IR-4.2.4
da
In Examples 14 and 15, the formula [NaCl] may be used to distinguish the molecular
compound consisting of one sodium atom and one chlorine atom from the solid with the
composition NaCl.
Examples:
1.
2.
3.
lR
Solid state structural information
na
IR-4.2.5
Na2CO3.10H2O
8H2S.46H2O
BMe3.NH3
ec
In the formulae of addition compounds, including multiple salts and solvates (particularly
hydrates), a special format is used. The proportions of constituents are indicated by arabic
numerals preceding the formulae of the constituents, and the formulae of the constituents are
separated by a centre dot. The rules for ordering the constituent formulae are described in
Section IR-4.4.3.5.
PA
C
Pr
ov
isi
o
Structural information can also be given by indicating structural type as a qualification of a
molecular formula (see Section IR-11.7.2). For example, polymorphs may be indicated by
adding in parentheses an abbreviated expression for the crystal system. Structures may also
be designated by adding the name of a type-compound in italics in parentheses, but such
usage may not be unambiguous. There are at least ten varieties of ZnS(h). Where several
polymorphs crystallise in the same crystal system they may be differentiated by the Pearson
symbol (see Sections IR-3.5.3 and IR-11.5.2). Greek letters are frequently employed to
designate polymorphs, but their use is often confused and contradictory and is not
recommended.
IU
Examples:
1.
2.
3.
TiO2(t) (anatase type)
TiO2(r) (brookite type)
AuCd(c), or AuCd (CsCl type)
For the formulae of solid solutions and non-stoichiometric phases, see Chapter IR-11.
Page 5 of 17
DRAFT 2 April 2004
6
IR-4.3
INDICATION OF IONIC CHARGE
Examples:
7.
2.
Cu2+
8.
3.
NO+
9.
4.
[Al(H2O)6]3+
10.
5.
H2NO3+
11.
6.
[PCl4]+
12.
13.
_
[P3O10]5 ,
_
[Fe(CN)6]4
_
[PW12O40]3
lR
na
O
O
P
isi
o
P
5-
O
O
O
P
or
O
O
ov
_
([CuCl3] )n, or
Pr
PA
C
IU
IR-4.4.1
_
_
or [O3POP(O)2OPO3]5 ,
O
14.
S 2O 72
ec
Cu+
O
IR-4.4
_
As3
_
HF2
_
CN
1.
O
om
me
n
da
tio
n
s
_
_
Ionic charge is indicated by means of a right upper index, as in An+ or An (not A+n or A n).
If the formula is placed in enclosing marks, the right upper index is placed outside the
enclosing marks. For polymeric ions, the charge of a single repeating unit should be placed
inside the parentheses that comprise the polymeric structure or the total charge of the
polymeric species should be placed outside the polymer parentheses. (The rules needed for
ordering the symbols in some of the example formulae below are given in Section IR-4.4.3.)
Cl
Cu
n-
Cl
Cl
n
SEQUENCE OF CITATION OF SYMBOLS IN FORMULAE
Introduction
Atomic symbols in formulae may be ordered in various ways. Section IR-4.4.3 describes the
conventions usually adopted for some important classes of compounds. As a prerequisite,
DRAFT 2 April 2004
Page 6 of 17
7
Ordering principles
IR-4.4.2.1
Electronegativity
tio
n
IR-4.4.2
s
Section IR-4.4.2 explains what is meant by the two ordering principles 'electronegativity' and
'alphabetical ordering'.
placed between chlorine and fluorine.
IR-4.4.2.2
Alphabetical order
om
me
n
da
If electronegativity is taken as the ordering principle in a formula or a part of a formula, the
atomic symbols are cited according to relative electronegativities, the least electronegative
element being cited first. For this purpose, Table VI* is used as a guide, except that oxygen is
A single letter symbol always precedes a two-letter symbol with the same initial letter, e.g. B
before Be, O before OH. The group NH4 is treated as a single symbol and so is listed after
ec
Na, for example.
na
lR
Where the entities to be arranged in a formula are polyatomic, the order of citation is decided
by selecting a particular atomic symbol to characterise the entity. The first symbol in the
formula of a polyatomic group, as written according to the appropriate rule in Section IR4.4.3, determines the alphabetical order. For example, C5H5, SCN, UO2, NO3, OH, and
[Zn(H2O)6]2+ are ordered under C, S, U, N, O, and Zn, respectively. If the first symbols are
the same, the symbol with the lesser right index is cited first, e.g. NO2 precedes N2O2. If this
isi
o
still does not discriminate, the subsequent symbols are used alphabetically and numerically to
define the order, e.g. NH2 precedes NO2 which precedes NO3.
Formulae for specific classes of compounds
PA
C
IR-4.4.3.
Pr
ov
To summarise and exemplify, the order of citation of some nitrogen-containing compounds
is:
_
_
_
_
_
_
N3 , NH2 , NH3, NO2 , NO3 , N2O22 , N3 .
IR-4.4.3.1
Binary compounds
IU
In accordance with established practice, the electronegativity criterion (Section IR-4.4.2.1) is
generally used in binary compounds.2
Examples:
*
Tables numbered with a Roman numeral are collected together at the end of this
book.
Page 7 of 17
DRAFT 2 April 2004
8
NH3
2.
3.
4.
5.
6.
H 2S
OF2
Cl2O
_
ClO
PH4+
7.
P 2O 74
8.
_
[SiAs4]8
9.
RbBr
11.
tio
n
da
_
om
me
n
10.
s
1.
_
[Re2Cl9]
_
_
HO or OH
12.
lR
13.
Rb15Hg16
Cu5Zn8 and Cu5Cd8
ec
_
Note that the formula for the hydroxide ion should be HO to be consistent with the above
convention.
IR-4.4.3.2
isi
o
na
Ordering by electronegativity could, in principle, be applied to ternary, quaternary, etc.
compounds. For most compounds consisting of more than two elements, however, other
criteria for ordering the element symbols in the formula are more often used (see Sections
IR-4.4.3.2 to IR-4.4.3.4).
(Formal) treatment as coordination compounds
Pr
ov
The nomenclature of coordination compounds is described in detail in Chapter IR-9. A brief
summary of the construction of formulae of coordination compounds is given here. Many
polyatomic compounds may conveniently be treated as coordination compounds for the
purpose of constructing a formula.
IU
PA
C
In the formula of a coordination entity, the symbol of the central atom(s) is/are placed first,
followed by the symbols or formulae of the ligands, unless additional structural information
can be presented by changing the order (see, for example, Section IR-4.4.3.3).
The order of citation of central atoms is based on electronegativity as described in Section IR4.4.2.1. Ligands are cited alphabetically (Section IR-4.4.2.2) according to the first symbol of
the ligand formula or ligand abbreviation (see Section IR-4.4.4) as written. Where possible,
the ligand formula should be written in such a way that a/the donor atom is closest to the
central atom to which it is attached.
DRAFT 2 April 2004
Page 8 of 17
9
5.
6.
7.
8.
9.
10.
11.
12.
13.
da
_
[PtBrCl(NH3)(NO2)]
[PtCl2(NH3)(py)]
[Co(en)F2(NH3)2]+, but [CoF2(NH2CH2CH2NH2)(NH3)2]+
_
[Co(NH3)5(N3)]2
na
14.
om
me
n
4.
ec
3.
PBrCl2
SbCl2F or [SbCl2F]
_
[Mo6O18]2
_
[CuSb2]5
[UO2]2+
_
[SiW12O40]4
_
[BH4]
_
[ICl4]
[PtCl2{P(OEt)3}2]
[Al(OH)(OH2)5]2+
lR
Examples:
1.
2.
tio
n
s
Square brackets may be used to enclose the whole coordination entity whether charged or not.
Established practice is always to use square brackets for coordination entities with a transition
metal as the central atom.
isi
o
In a few cases, a moiety which comprises different atoms and which occurs in a series of
compounds is considered as an entity that acts as a central atom and is cited as such, even if
this violates the alphabetical order of ligands. For example, PO and UO2 are regarded as
ov
single entities in Examples 15 and 16.
POBr3 (alphabetically, PBr3O)
[UO2Cl2] (alphabetically, [UCl2O2])
Pr
Examples:
15.
16.
IU
PA
C
For derivatives of parent hydrides, the alphabetical order of ligands is traditionally disobeyed
in that remaining hydrogen atoms are listed first among the ligands in the formula.
Page 9 of 17
Examples:
17.
18.
19.
GeH2F2
SiH2BrCl
B2H5Cl
DRAFT 2 April 2004
10
For carbaboranes, there has previously been some uncertainty over the order of B and C.3
(recommended)
(recommended)
da
B3C2H5
B3C2H4Br
om
me
n
Examples:
20.
21.
tio
n
s
The order 'B before C' recommended here conforms to both electronegativity and
alphabetical order (i.e. it is an exception to the Hill order1 in Section IR-4.2.1). In addition,
carbon atoms that replace skeletal boron atoms are cited immediately after boron, regardless
of what other elements are present. (See also Section IR-6.2.4.4).
25.
IR-4.4.3.3
isi
o
26.
lR
24.
HNO3 (traditional) or [NO2(OH)] (coordination)
H2PHO3 (traditional) or [PHO(OH)2] (coordination)
_
_
H2PO4 (traditional) or [PO2(OH)2]
(coordination)
H5P3O10 (traditional) or
[(HO)2P(O)OP(O)(OH)OP(O)(OH)2] (coordination)
(HBO2)n (traditional) or (B(OH)O)n (coordination)
na
Examples:
22.
23.
ec
For inorganic oxoacids, there is a traditional ordering of formulae in which the 'acid' or
'replaceable' hydrogen atoms (hydrogen atoms bound to oxygen) are listed first, followed by
the central atom, then 'non-replaceable' hydrogen atoms (hydrogen atoms bound directly to
the central atom), and finally oxygen. This format is an alternative to writing the formulae as
coordination compound formulae (see Section IR-8.3).
Chain compounds
PA
C
Pr
ov
For chain compounds containing three or more different elements, the sequence of atomic
symbols should generally be in accord with the order in which the atoms are bound in the
molecule or ion, rather than using alphabetical order or order based on electronegativity.
However, if one wishes to view a compound formally as a coordination compound, e.g. in
connection with a discussion of additive naming of the compound, one may use a
coordination-compound type of formula, as in Example 1 below.
IU
Examples:
1.
2.
3.
4.
5.
_
_
_
SCN (not CNS ) = [C(N)S] , nitridosulfidocarbonate(1-)
BrSCN (not BrCNS)
HOCN (cyanic acid)
HONC (fulminic acid)
_
[O3POSO3]3
DRAFT 2 April 2004
Page 10 of 17
11
IR-4.4.3.4
Generalised salt formulae
om
me
n
da
tio
n
s
If the formula of a compound containing three or more elements is not naturally assigned
using the preceding two sections, the compound can be treated as a generalised salt. This
term is taken to mean any compound in which it is possible to identify at least one
constituent which is a positive ion or can be classified as electropositive or more
electropositive than the other constituents, and at least one constituent which is a negative ion
or can be classified as electronegative or more electronegative than the rest of the
constitutents. The ordering principle is then:
(i) all electropositive constituents precede all electronegative constituents;
(ii) within each of the two groups of constituents, alphabetical order is used.
lR
ec
MgCl(OH)
FeO(OH)
NaTl(NO3)2
Li[H2PO4]
NaNH4[HPO4]
Na[HPHO3]
CuK5Sb2 or K5CuSb2
K5[CuSb2]
H[AuCl4]
Na(UO2)3[Zn(H2O)6](O2CMe)9
na
2.
3.
4.
5.
6.
7.
8.
9.
10.
11
KMgF3
isi
o
Examples:
1.
PA
C
Pr
ov
The first formula in Example 8 was arrived at by considering K and Cu to be electropositive
constituents and Sb to be electronegative, the second by considering K to be electropositive
and Cu and Sb to be electronegative. No structural information is conveyed by these
formulae. The formula in Example 9, on the other hand, implies the presence of a molecular
_
entity or coordination entity [CuSb2]5 .
IU
Deviation from alphabetical order of constituents in the same class is allowed to emphasise
similarities between compounds.
Example:
12.
CaTiO3 and ZnTiO3 (rather than TiZnO3)
Some generalised salts may also be treated as addition compounds, see Section IR-4.4.3.5.
Page 11 of 17
DRAFT 2 April 2004
12
Addition compounds, multiple salts and solvates
s
IR-4.4.3.5
IR-4.4.4
om
me
n
ec
3CdSO4.8H2O
Na2CO3.10H2O
Al2(SO4)3.K2SO4.24H2O
AlCl3.4EtOH
8H2S.46H2O
C6H6.NH3.Ni(CN)2
BF3.2H2O
BF3.2MeOH
lR
Examples:
1.
2.
3.
4.
5.
6.
7.
8.
da
tio
n
In the formulae of addition compounds, multiple salts and solvates, the component molecules
or entities are cited in order of increasing number; if they occur in equal numbers, they are
cited in alphabetical order of the first symbols. In addition compounds containing water, the
water remains conventionally cited last. However, boron compounds are no longer treated as
exceptions.
Ligand abbreviations
ov
isi
o
na
Since abbreviations are widely used in the chemical literature, agreement on their use and
meaning is desirable. This Section provides guidelines for the selection of ligand
abbreviations for application in the formulae of coordination compounds (Section IR9.2.3.4). Some commonly used ligand abbreviations are listed in Table VII with diagrams of
most of the ligands shown in Table VIII.
Pr
An abbreviation for an organic ligand should be derived from a name consistent with the
current rules for the systematic nomenclature of organic compounds.4 (For some ligands a
IU
PA
C
non-systematic name is included in Table VII if it was the source of the abbreviation and if
that abbreviation is still commonly used). However, new abbreviations should be constructed
according to the following recommendations:
(i)
Ligand abbreviations should be formulated so as to avoid confusion and
misunderstanding. Since a reader may not be familiar with an abbreviation, it should be
explained at the beginning of the text or when it first appears in a publication.
(ii)
New meanings should not be suggested for abbreviations or acronyms that have
generally accepted meanings, e.g. DNA, NMR, ESR, HPLC, Me (for methyl), Et (for ethyl),
etc.
DRAFT 2 April 2004
Page 12 of 17
13
An abbreviation should readily suggest the ligand name, e.g. ida for iminodiacetato.
s
(iii)
The use of non-systematic names (and their abbreviations) is discouraged.
da
(v)
tio
n
(iv)
Abbreviations should be as short as practicable, but should contain more than one
letter or symbol.
om
me
n
(vi)
Abbreviations should normally use only lower-case letters, with several well
established exceptions:
(a) abbreviations for alkyl, aryl and similar groups, which have the first letter
capitalised with the remaining letters in lower case, e.g. Me (for methyl), Ac (for
acetyl), Cp (for cyclopentadienyl), etc.;
ec
(b) abbreviations containing atomic symbols, e.g. [12]aneS4;
(c) abbreviations containing Roman numerals, e.g. H2ppIX for protoporphyrin IX;
lR
(d) abbreviations for ligands containing readily removable hydrons (see vii).
isi
o
na
(N.B. Abbreviations for solvents that behave as ligands should also be in lower case letters
[e.g. dmso for dimethyl sulfoxide{(methylsulfinyl)methane}, thf for tetrahydrofuran]; the
practice of capitalising the abbreviation of a solvent when it does not behave as a ligand is
strongly discouraged as an unnecessary distinction).
ov
(vii)
Hydronation of anionic ligands, e.g. ida, leads to acids which may be abbreviated by
the addition of H, e.g. Hida, H2ida.
IU
IR-4.5
PA
C
Pr
(viii) Ligands which are normally neutral, but which continue to behave as ligands on
losing one or more hydrons, are abbreviated by adding _1H, _2H, etc. as subscripts
(including the numeral 1) after the usual abbreviation of the ligand. For example, if
_
Ph2PCH2PPh2 (dppm) loses one hydron to give [Ph2PCHPPh2] its abbreviation is
dppm_lH; if it loses two hydrons, its abbreviation is dppm_2H, etc.
IR-4.5.1
ISOTOPICALLY MODIFIED COMPOUNDS.5
General formalism
The mass number of any specific nuclide can be indicated in the usual way with a left
superscript preceding the appropriate atomic symbol (see Section IR-3.2).
Page 13 of 17
DRAFT 2 April 2004
14
Isotopically substituted compounds
da
IR-4.5.2
tio
n
s
When it is necessary to cite different nuclides at the same position in a formula, the nuclide
symbols are written in alphabetical order; when their atomic symbols are identical the order is
that of increasing mass number. Isotopically modified compounds may be classified as
isotopically substituted compounds and isotopically labelled compounds.
H3HO
H36Cl
235UF6
42KNa14CO3
5.
6.
7.
Isotopically labelled compounds
IR-4.5.3.1
Types of labelling
K[32PF6]
K342K[Fe(14CN)6]
na
lR
IR-4.5.3
32PCl3
ec
Examples:
1.
2.
3.
4.
om
me
n
An isotopically substituted compound has a composition such that all the molecules of the
compound have only the indicated nuclide(s) at each designated position. The substituted
nuclides are indicated by insertion of the mass numbers as left superscripts preceding the
appropriate atom symbols in the normal formula.
Specifically labelled compounds
Pr
IR-4.5.3.2
ov
isi
o
An isotopically labelled compound may be considered formally as a mixture of an
isotopically unmodified compound and one or more analogous isotopically substituted
compounds. They may be divided into several different types. Specifically labelled
compounds and selectively labelled compounds are treated here.
IU
PA
C
An isotopically labelled compound is called a specifically labelled compound when a unique
isotopically substituted compound is added formally to the analogous isotopically
unmodified compound. A specifically labelled compound is indicated by enclosing the
appropriate nuclide symbol(s) and multiplying subscript (if any) in square brackets.
IR-4.5.3.3
Examples:
1.
2.
3.
H[36Cl]
[32P]Cl3
[15N]H2[2H]
4.
5.
6.
[13C]O[17O]
[32P]O[18F3]
Ge[2H2]F2
Selectively labelled compounds
DRAFT 2 April 2004
Page 14 of 17
15
da
[36Cl]SOCl2
[2H]PH3
[10B]B2H5Cl
om
me
n
Examples:
1.
2.
3.
tio
n
s
A selectively labelled compound may be considered as a mixture of specifically labelled
compounds. It is indicated by prefixing the formula by the nuclide symbol(s) preceded by
any necessary locant(s) (but without multiplying subscripts) enclosed in square brackets.
The number of possible labels for a given position may be indicated by subscripts separated
by semicolons added to the atomic symbol(s) in the isotopic descriptor.
Example:
4.
[1-2H1;2]SiH3OSiH2OSiH3
OPTIONAL MODIFIERS OF FORMULAE
IR-4.6.1
Oxidation state
lR
ec
IR-4.6
1.
4.
K[OsVIII(N)O3]
5.
PbII2PbIVO4
_
Na2O I2
_
[MoV2MoVI4O18]2
6.
[Os0(CO)5]
Pr
2.
_
[PV2Mo18O62]6
ov
Examples:
isi
o
na
The oxidation state of an element in a formula may be indicated by an oxidation number
written as a right upper index (superscript) in Roman numerals. Oxidation state zero may be
represented by the numeral 0 but is not usually shown. If an element occurs with more than
one oxidation state in the same formula, the element symbol is repeated, each symbol being
assigned a number cited in sequence in increasing magnitude and from negative to positive.
3.
IU
PA
C
Where it is not feasible or reasonable to define an oxidation state for each individual member
of a group (or cluster), the overall oxidation level of the group should be defined by a formal
ionic charge, indicated as in Section IR-4.3. This avoids the use of fractional oxidation states.
IR-4.6.2
Page 15 of 17
Examples:
7.
O2
_
8.
Formulae of radicals
DRAFT 2 April 2004
Fe4S43+
16
da
tio
n
s
A radical is an atom or molecule with one or more unpaired electrons. It may have positive,
negative or zero charge. An unpaired electron may be indicated in a formula by a superscript
dot. The dot is placed as a right upper index to the chemical symbol, so as not to interfere
with indications of mass number, atomic number or composition. In the case of diradicals,
etc., the superscript dot is preceded by the appropriate superscript multiplier. The radical dot
with its multiplier, if any, precedes any charge. To avoid confusion, the multiplier and the
radical dot can be placed within parentheses.
O 2•−
BH 3•+
PO3•2−
NO(2•)−
ec
5.
6.
7.
8.
Formulae of optically active compounds
na
IR-4.6.3
H•
HO •
NO 2•
O 22•
lR
Examples:
1.
2.
3.
4.
om
me
n
Metals and their ions or complexes often possess unpaired electrons but, by convention, they
are not considered to be radicals, and radical dots are not used in their formulae. However,
there may be occasions when a radical ligand is bound to a metal or metal ion where it is
desirable to use a radical dot.
isi
o
The sign of optical rotation is placed in parentheses, the wavelength (in nm) being indicated
as a right subscript. The whole symbol is placed before the formula and refers to the sodium
Indication of excited states
PA
C
IR-4.6.4
(+)589[Co(en)3]Cl3
(_)589[Co{(_)NH2CH(CH3)CH2NH2}3]Cl3
Pr
Examples:
1.
2.
ov
D-line unless otherwise stated.
IU
Excited electronic states may be indicated by an asterisk as right superscript. This practice
does not differentiate between different excited states.
IR-4.6.5
Examples:
1.
2.
He*
NO*
Structural descriptors
DRAFT 2 April 2004
Page 16 of 17
17
cis-[PtCl2(NH3)2]
trans-[PtCl4(NH3)2]
da
Examples:
1.
2.
tio
n
s
Structural descriptors such as cis-, trans-, etc., are listed in Table V. Usually such descriptors
are used as italicised prefixes and are connected to the formula by a hyphen.
Example:
3.
[(H3N)5Cr(µ-OH)Cr(NH3)5]5+
REFERENCES
4.
ec
IU
PA
C
Pr
ov
5.
lR
3.
This is the so-called Hill order. See, E.A. Hill, J. Amer. Chem. Soc., 22, 479 (1900).
For intermetallic compounds, earlier recommendations prescribed alphabetical ordering
rather than by electronegativity (see Section I-4.6.6 of Nomenclature of Inorganic
Chemistry, Recommendations 1990, Blackwell Scientific Publications, Oxford, 1990).
For example, the ordering of B and C in formulae was inconsistent in Nomenclature of
Inorganic Chemistry, Recommendations 1990, Blackwell Scientific Publications,
Oxford, 1990.
Title of New Blue Book needed to replace:Nomenclature of Organic Chemistry,
1979 edition, p. 480, Rule E-4.4.
Chapter II-2 of Nomenclature of Inorganic Chemistry II, Recommendations 2000,
Royal Society of Chemistry, 2001.
na
1.
2.
isi
o
IR-4.8
om
me
n
The descriptor µ designates an atom or group bridging coordination centres.
Page 17 of 17
DRAFT 2 April 2004
1
Compositional Nomenclature and Overview of Names of
Ions and Radicals (Draft March 2004)
s
IR-5
tio
n
CONTENTS
Pr
ov
isi
o
na
lR
ec
om
me
n
da
IR-5.1 Introduction
IR-5.2 Stoichiometric names of elements and binary compounds
IR-5.3 Names of ions
IR-5.3.1 General
IR-5.3.2 Cations
IR-5.3.2.1 General
IR-5.3.2.2 Monoatomic cations
IR-5.3.2.3 Homopolyatomic cations
IR-5.3.2.4 Heteropolyatomic cations
IR-5.3.3 Anions
IR-5.3.3.1 Overview
IR-5.3.3.2 Monoatomic anions
IR-5.3.3.3 Homopolyatomic anions
IR-5.3.3.4 Heteropolyatomic anions
IR-5.4 Generalised stoichiometric names
IR-5.4.1 Electropositive and electronegative constituents; order of citation
IR-5.4.2 Indication of proportions of constituents
IR-5.4.2.1 Use of multiplicative prefixes
IR-5.4.2.2 Use of charge and oxidation numbers
IR-5.4.2.3 Multiple monoatomic constituents vs. homopolyatomic
constituents
IR-5.5 Names of addition compounds
IR-5.6 Final remarks
IR-5.7 References
INTRODUCTION
PA
C
IR-5.1
IU
Compositional nomenclature is formally based on composition, not structure, and may thus
be the (only) choice if little or no structural information is available or a minimum of
structural information is to be conveyed.
Page 1 of 16
The simplest type of compositional name is a stoichiometric name, which is just a reflection
of the empirical formula (Section IR-4.2.1) or the molecular formula (Section IR-4.2.2) of
DRAFT 2 April 2004
2
s
the compound. In stoichiometric names, proportions of constituent elements may be indicated
in several ways, using numerical prefixes, oxidation numbers or charge numbers.
IR-5.2
STOICHIOMETRIC
COMPOUNDS
NAMES
OF
om
me
n
da
tio
n
In some cases, a compound may be viewed as composed of constituents that may themselves
be given names of any of several types (including stoichiometric names), and the overall
name of the compound is then assembled from the names of the constituents so as to indicate
the proportions of the constituents. One category of such compositional names is
generalised stoichiometric (see Section IR-5.4) in which the various parts may themselves be
names of mono- and polyatomic ions. For this reason, Section IR-5.3, devoted to the naming
of ions, is included. Another category consists of the names devised for addition compounds
which have a format of their own, described in Section IR-5.5.
ELEMENTS
AND
NEUTRAL
BINARY
ec
A purely stoichiometric name carries no information about the structure of the compound.
lR
This section deals with stoichiometric names of neutral compounds. Stoichiometric names of
ions are described in Section IR-5.3.
na
In the simplest case, the species to be named consists of only one element, and the name is
formed by adding the relevant multiplicative prefix to the element name (e.g. S8, octasulfur).
isi
o
This case is dealt with more fully in Sections IR-3.4.2 and IR-3.5.2.
ov
When constructing a stoichiometric name for a binary compound, one element is designated
as the electropositive constituent and the other the electronegative constituent. The
electropositive constituent is by convention the element that occurs last in the sequence of
Table VI* (except that oxygen is placed between chlorine and fluorine) and its name is the
IU
PA
C
Pr
unmodified element name (Table I). The name of the electronegative constituent is
constructed by modifying the element name with the ending 'ide', as explained in detail for
monoatomic anions in Section IR-5.3.3.2. All element names thus modified with the 'ide'
ending are given in Table IX.
The stoichiometric name of the compound is then formed by combining the name of the
electropositive constituent, cited first, with that of the electronegative constituent, both suitably
qualified by any necessary multiplicative prefixes ('mono', 'di', 'tri', 'tetra', 'penta', etc., given in
Table IV). The numerical prefixes precede the names they multiply, and are joined directly to
them without spaces or hyphens. The final vowels of numerical prefixes should not be elided
*
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
Page 2 of 16
3
s
(although monoxide, rather than monooxide, is an allowed exception through general use).
The two parts of the name are separated by a space.
tio
n
Stoichiometric names may correspond to the empirical formula or to a molecular formula
different from the empirical formula (compare Examples 3 and 4 below).
HCl
NO
NO2
N 2O 4
Cl2O
ClO2
Fe3O4
hydrogen chloride
nitrogen oxide, or nitrogen monooxide, or nitrogen monoxide
nitrogen dioxide
dinitrogen tetraoxide
dichlorine oxide
chlorine dioxide
triiron tetraoxide
8.
9.
10.
SiC
SiCl4
Ca3P2
silicon carbide
silicon tetrachloride
tricalcium diphosphide, or calcium phosphide
11.
12.
13.
NiSn
Cu5Zn8
Cr23C6
nickel stannide
pentacopper octazincide
tricosachromium hexacarbide
na
lR
ec
om
me
n
da
Examples:
1.
2.
3.
4.
5.
6.
7.
isi
o
Multiplicative prefixes need not be used in binary names if there is no ambiguity about the
stoichiometry of the compound (such as in Example 10 above). The prefix 'mono' is, strictly
speaking, superfluous and is only needed for emphasizing stoichiometry when discussing
compositionally related substances, such as Examples 2, 3 and 4 above.
Pr
ov
Alternatively, proportions of constituents may be indicated by using oxidation numbers or
charge numbers (Section IR-5.4.2).
IR-5.3
PA
C
For compounds containing more than two elements, further conventions are required to form
a compositional name (see Sections IR-5.4 and IR-5.5).
IU
IR-5.3.1
Page 3 of 16
NAMES OF IONS
General
The charges of the atoms need not be specified in a stoichiometric name. In many cases,
however, atoms or groups of atoms are known to carry a particular charge. Within
compositional nomenclature, the name of a compound can include the names of individual
DRAFT 2 April 2004
4
IR-5.3.2.1
General
tio
n
Cations
da
IR-5.3.2
s
such ions constructed as stoichiometric names or according to other principles, as described
below.
IR-5.3.2.2
om
me
n
A cation is a monoatomic or polyatomic species having one or more positive charges. The
charge on a cation can be indicated in names and formulae by using the charge number or, in
the case of additively named cations, by the oxidation number(s) of the central atom or atoms.
Oxidation and charge numbers are discussed in Section IR-5.4.2.2.
Monoatomic cations
Examples:
1.
lR
ec
The name of a monoatomic cation is that of the element with an appropriate charge number
appended in parentheses. Unpaired electrons in monoatomic cations may be indicated using a
radical dot, i.e. a centred dot placed in parentheses after the name, with a numerical prefix if
necessary.
Na+
na
isi
o
PA
C
Pr
ov
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
sodium(l+)
Cr3+ chromium(3+)
Cu+ copper(1+)
Cu2+ copper(2+)
I+
iodine(1+)
H+
hydrogen(1+), hydron
1H +
protium(1+), proton
2H +
deuterium(1+), deuteron
3H +
tritium(1+), triton
+
•
helium(•1+)
He
O •+ oxygen(•1+)
The names of the hydrogen isotopes are discussed in Section IR-3.3.2.
IU
IR-5.3.2.3
Homopolyatomic cations
Homopolyatomic cations are named by adding the charge number to the stoichiometric name
of the corresponding neutral species, i.e. the element name with the appropriate numerical
prefix. A radical dot or radical dots may be added to indicate the presence of unpaired
electrons.
DRAFT 2 April 2004
Page 4 of 16
da
dioxygen(1+) or dioxygen(•1+)
tetrasulfur(2+)
dimercury(2+)
pentabismuth(4+)
trihydrogen(1+)
Heteropolyatomic cations
om
me
n
IR-5.3.2.4
O2+ or O2•+
S42+
Hg22+
Bi54+
H 3+
tio
n
Examples:
1.
2.
3.
4.
5.
s
5
Heteropolyatomic cations are usually named either substitutively (see Section IR-6.4) or
additively (see Chapter IR-7). Substitutive names do not require a charge number, because
the name itself implies the charge (Examples 2 and 4 below). A radical dot or radical dots
may be added to an additive name to indicate the presence of unpaired electrons.
NH4+
3.
4.
5.
PH4+
systematic)
oxidanium (substitutive), or oxonium (allowed non-
isi
o
BH3•+
ov
6.
H4O2+
SbF4+
lR
2.
H 3O +
azanium (substitutive), or ammonium (allowed non-
systematic)
phosphanium (substitutive)
oxidanediium (substitutive)
tetrafluorostibanium (substitutive), or
na
Examples:
1.
ec
A few cations have established and still allowed non-systematic names.
tetrafluoridoantimony(1+) or tetrafluoridoantimony(V)
(both additive)
boraniumyl (substitutive) or trihydridoboron(•1+) (additive)
Anions
PA
C
IR-5.3.3
Pr
More examples are given in Table IX.
IU
IR-5.3.3.1
Page 5 of 16
Overview
An anion is a monoatomic or polyatomic species having one or more negative charges. The
charge on an anion can be indicated in the name by using the charge number or, in the case of
an additively named anion, by the oxidation number(s) of the central atom or atoms.
Oxidation and charge numbers are discussed in Section IR-5.4.2.2.
DRAFT 2 April 2004
6
Examples:
2.
3.
4.
5.
6.
[CoCl4]
_
NO2
chloride(1_), or chloride
disulfide(2_)
phosphanide
phosphanediide
2_
tetrachloridocobaltate(2_), or tetrachloridocobaltate(II)
dioxidonitrate(1_), or nitrite
Monoatomic anions
ec
IR-5.3.3.2
_
Cl
_
S 22
_
PH2
_
PH2
om
me
n
1.
da
tio
n
s
The endings in anion names are 'ide' (monoatomic or homopolyatomic species,
heteropolyatomic species named from the parent hydride), 'ate' (heteropolyatomic species
named additively), and 'ite' (used in a few names which are still allowed but do not derive
from current systematic nomenclature). When there is no ambiguity, the charge number may
be omitted, as in Example 1 below. Parent hydride-based names do not carry charge
numbers, because the name itself implies the charge (Examples 3 and 4 below).
isi
o
ov
chlorine, chloride
carbon, carbide
xenon, xenonide
tungsten, tungstide
bismuth, bismuthide
Pr
Examples:
1.
2.
3.
4.
5.
na
lR
The name of a monoatomic anion is the element name (Table I) modified so as to carry the
anion designator 'ide', either formed by replacing the ending of the element name ('en', 'ese',
'ic', 'ine', 'ium', 'ogen', 'on', 'orus', 'um', 'ur', 'y', or 'ygen') by 'ide' or by directly adding 'ide' as an
ending to the element name.
PA
C
In one case, an abbreviated name has to be chosen: germanium, germide. The systematic
_
name 'germanide' designates the anion GeH3 .
IU
Some names of monoatomic anions are based on the root of the Latin element names. In
these the ending 'um' or 'ium' is replaced by 'ide'.
Examples:
6.
7.
8.
silver, argentum, argentide
gold, aurum, auride
copper, cuprum, cupride
DRAFT 2 April 2004
Page 6 of 16
7
s
iron, ferrum, ferride
lead, plumbum, plumbide
tin, stannum, stannide
sodium, natrium, natride
potassium, kalium, kalide
tio
n
9.
10.
11.
12.
13.
da
All element names thus modified are included in Table IX.
om
me
n
Charge numbers and radical dots may be added as appropriate to specify the anion fully.
Examples:
14.
15.
16.
oxide(2_), or oxide
oxide(•1−)
nitride(3_), or nitride
Homopolyatomic anions
ec
IR-5.3.3.3
_
O2
O •−
_
N3
na
lR
Homopolyatomic anions are named by adding the charge number to the stoichiometric name
of the corresponding neutral species, i.e. the element name with the appropriate numerical
prefix. Again, a radical dot or dots may be added as appropriate.
isi
o
In a few cases, non-systematic names are still allowed alternatives.
Examples:
ov
1.
2
_
O3
_
I3
Cl2•−
_
C22
_
N3
_
S 22
_
Sn52
_
Pb94
Pr
2.
Systematic name
O2 or O2•− dioxide(1_) or
dioxide(•1−)
_
dioxide(2_)
O 2
3.
PA
C
4.
5.
6.
7.
IU
8.
Page 7 of 16
Alternative name
_
9.
10.
trioxide(1_)
triiodide(1_)
dichloride(•1−)
dicarbide(2_)
trinitride(1_)
disulfide(2_)
pentastannide(2_)
nonaplumbide(4_)
DRAFT 2 April 2004
superoxide
peroxide
ozonide
acetylide
azide
8
s
In some cases, homopolyatomic anions may be considered as derived from a parent hydride
by removal of hydrons (see Section IR-6.4).
11.
dioxidanediide
disulfanediide
Heteropolyatomic anions
om
me
n
IR-5.3.3.4
_
O 22
_
S 22
da
10.
tio
n
Examples:
Heteropolyatomic anions are usually named either substitutively (see Section IR-6.4.4) or
additively (see Chapter IR-7 and Section IR-9.2.2). A radical dot, or dots, may be added to
additive names to indicate the presence of unpaired electron(s).
A few heteropolyatomic anions have established and still allowed non-systematic names.
Examples:
NH2
_
azanide (substitutive), dihydridonitrate(1_) (additive),
ec
1.
or amide (allowed non-systematic)
2.
GeH3
3.
HS
4.
H 3S
5.
H 2S•−
6.
SO32
lR
_
germanide (substitutive), or
trihydridogermanate(1_) (additive)
_
na
sulfanide (substitutive), or hydridosulfate(1_) (additive)
isi
o
_
7.
ov
_
8.
_
ClO3
oxidochlorate(1_) (additive), or
hypochlorite (allowed non-systematic)
trioxidochlorate(1_) (additive), or
Pr
PA
C
IU
sulfanuidyl or λ4-sulfanidyl (both substitutive),
or dihydridosulfate(•1−) (additive)
trioxidosulfate(2_) (additive), or sulfite (allowed nonsystematic)
_
ClO
chlorate (allowed non-systematic)
_
9.
[PF6]
10.
_
[CuCl4]2
11.
sulfanuide or λ4-sulfanide (both substitutive),
or trihydridosulfate(1_) (additive)
hexafluoro-λ5-phosphanuide (substitutive), or
hexafluoridophosphate(1_) (additive)
[Fe(CO)4]
2_
tetrachloridocuprate(II) (additive)
tetracarbonylferrate(_II) (additive)
All allowed, but not fully systematic, anion names are given in Table IX.
DRAFT 2 April 2004
Page 8 of 16
9
tio
n
s
Note that in Ref. 1, radical anions consisting of only hydrogen and one other element were
named additively using the ending 'ide' rather than the ending 'ate' (e.g. Example 5 above).
Making this exception to the general system of additive nomenclature for these particular
cases is now discouraged.
om
me
n
da
When one or more hydron(s) are attached to an anion at an unknown position(s), or at a
position(s) which one cannot or does not wish to specify, a 'hydrogen name' (see Section IR8.4) may be used. Such names may also be used for simpler compounds, such as partially
dehydrogenated oxoacids. Certain of these names have accepted abbreviated forms, such as
hydrogencarbonate, dihydrogenphosphate, etc. All such accepted abbreviated names are given
in Section IR-8.5.
Examples:
12.
13.
_
hydrogen(nonadecaoxidohexamolybdate)(1_)
hydrogen(trioxidocarbonate)(1_), or hydrogencarbonate
dihydrogen(tetraoxidophosphate)(1_),
ec
14.
HMo6O19
_
HCO3
_
H2PO4
lR
or dihydrogenphosphate
GENERALISED STOICHIOMETRIC NAMES
IR-5.4.1
Electropositive and electronegative constituents; order of citation
na
IR-5.4
ov
isi
o
The constituents of the compound to be named are divided into formally electropositive and
formally electronegative constituents. There must be at least one electropositive and one
electronegative constituent. Cations are electropositive and anions electronegative, by
definition. Electropositive elements occur later in Table VI than electronegative elements,
except that oxygen is placed between chlorine and fluorine by custom.
IU
PA
C
Pr
In principle, the division into electropositive and electronegative constituents is arbitrary if the
compound contains more than two elements. In practice, however, there is often no problem
in deciding where the division lies.
The names of the electropositive constituents precede those of the electronegative constituents
in the overall name. The order of citation is alphabetical within each class of constituents
(multiplicative prefixes being ignored), except that hydrogen is always cited last among
electropositive constituents if actually classified as an electropositive constituent.
This principle for constructing generalised stoichiometric names parallels the principle for
constucting 'generalised salt formulae' in Section IR-4.4.3.4. However, the order of citation in
Page 9 of 16
DRAFT 2 April 2004
10
s
a generalised stochiometric name is not necessarily the same as the order of symbols in the
corresponding generalised salt formula, as is seen from Examples 5 and 7 below.
da
IBr
iodine bromide
PBrClI
phosphorus bromide chloride iodide
ArHF or ArFH
argon hydrogen fluoride, or argon fluoride hydride
ClOF or ClFO
chlorine oxygen fluoride or chlorine fluoride oxide
CuK5Sb2 or K5CuSb2
om
me
n
Examples:
1.
2.
3.
4.
5.
tio
n
The following generalised stoichiometric names, based only on single-element constituents,
do not carry information about the structure.
copper pentapotassium diantimonide, or
pentapotassium cupride diantimonide
na
lR
ec
Note from these examples that the order of any two elements in the name depends on the
arbitrary division of elements into electropositive and electronegative constituents. (The same
applies to the order of the element symbols in the formulae as illustrated in Section IR4.4.3.4). Additive names representing the actual structure of the compounds in Examples 3
and 4 (FArH and FClO, respectively) are given in Section IR-7.2.
ov
Cl2O2+
(dichlorine dioxide)(1+)
Pr
Example:
6.
isi
o
In some cases, the use of substitutive or additive nomenclature for naming an ion is not
possible or desirable because of the lack of structural information. In such cases, it may be
best to give a stoichiometric name and add the charge number. Parentheses are needed to
make it clear that the charge number is associated with the entire compound.
PA
C
However, when names of polyatomic ions occur as constituents in a generalised
stoichiometric name, a certain amount of structural information is often implied by the name.
Example:
7.
NaNH4[HPO4]
ammonium sodium hydrogenphosphate
Indication of proportions of constituents
IR-5.4.2.1
Use of multiplicative prefixes
IU
IR-5.4.2
DRAFT 2 April 2004
Page 10 of 16
11
disodium trioxidocarbonate, or sodium carbonate
tetrapotassium hexacyanidoferrate
phosphorus trichloride oxide
magnesium potassium trichloride
da
Na2CO3
K4[Fe(CN)6]
PCl3O
KMgCl3
om
me
n
Examples:
1.
2.
3.
4.
tio
n
s
The proportions of the constituents, be they monoatomic or polyatomic, may be indicated in
generalised stoichiometric names by numerical prefixes, as was the case for the constituents
of binary compounds (Section IR-5.2).
When the name of the constituent itself starts with a multiplicative prefix (as in disulfate,
dichromate, triphosphate, tetraborate, etc.), or when ambiguity could otherwise arise, the
alternative multiplicative prefixes 'bis', 'tris', 'tetrakis', 'pentakis', etc. (Table IV) are used and
the name of the group acted upon by the alternative prefix is placed in parentheses.
IR-5.4.2.2
lR
ec
calcium bis(trioxidonitrate), or calcium nitrate
bis(dioxidouranium) tetraoxidosulfate
barium bis(tetrafluoridobromate)
uranium bis(disulfate)
tricalcium bis(phosphate)
calcium diphosphate
calcium bis(hydrogencarbonate)
na
Ca(NO3)2
(UO2)2SO4
Ba[BrF4]2
U(S2O7)2
Ca3(PO4)2
Ca2P2O7
Ca(HCO3)2
isi
o
Examples:
5.
6.
7.
8.
9.
10.
11.
Use of charge and oxidation numbers
IU
PA
C
Pr
ov
It is possible to provide information on the proportions of the constituents in names by using
one of two other devices: the charge number, which designates ionic charge, and the oxidation
number, which designates oxidation state. In nomenclature, the use of the charge number is
preferred as the determination of the oxidation number is sometimes ambiguous and
subjective. It is therefore advisable to use oxidation numbers only when there is no
uncertainty about their assignment.
Page 11 of 16
The charge number is a number whose magnitude is the ionic charge. It is written in
parentheses immediately after the name of an ion, without a space. The charge is written in
arabic numerals, followed by the sign of the charge. Note that unity (1) is always indicated,
unlike in superscript charge designations (which are used in formulae). No charge number is
used after the name of a neutral species.
DRAFT 2 April 2004
12
s
iron(2+) sulfate
iron(3+) sulfate
dioxidouranium(1+) sulfate
dioxidouranium(2+) sulfate
potassium hexacyanidoferrate(4_)
hexaamminecobalt(3+) chloride sulfate
da
FeSO4
Fe2(SO4)3
(UO2)2SO4
UO2SO4
K4[Fe(CN)6]
[Co(NH3)6]Cl(SO4)
tio
n
Examples:
1.
2.
3.
4.
5.
6.
phosphorus(V) chloride
sodium pentacarbonylmanganate(_I)
pentacarbonyliron(0)
ec
PCl5
Na[Mn(CO)5]
[Fe(CO)5]
lR
Example:
7.
8.
9.
om
me
n
The oxidation number (see Sections IR-4.6.1 and IR-9.1.2.8) of an element is indicated by a
Roman numeral placed in parentheses immediately following the name (modified by the
ending 'ate' if necessary) of the element to which it refers. The oxidation number may be
positive, negative or zero (represented by the numeral 0). An oxidation number is always
non-negative unless the minus sign is explicitly used (the positive sign is never used). Nonintegral oxidation numbers are not used for nomenclature purposes.
isi
o
na
Several conventions are observed for inferring oxidation numbers, the use of which is
particularly common in the names of compounds of transition elements. Hydrogen is
considered positive (oxidation number I) in combination with non-metallic elements and
negative (oxidation number _I) in combination with metallic elements. Organic groups
combined with metal atoms are treated sometimes as anions (for example, a methyl ligand is
_
usually considered to be a methanide ion, CH3 ), sometimes as neutral (e.g. carbon
Pr
ov
monooxide). Bonds between atoms of the same species make no contribution to oxidation
number.
Examples:
10.
11.
12.
13.
NO2
Fe3O4
MnO2
nitrogen(I) oxide
nitrogen(IV) oxide
iron(II) diiron(III) oxide
manganese(IV) oxide
14.
15.
16.
17.
18.
CO
FeSO4
Fe2(SO4)3
SF6
(UO2)2SO4
carbon(II) oxide
iron(II) sulfate
iron(III) sulfate
sulfur(VI) fluoride
dioxidouranium(V) sulfate
PA
C
IU
N 2O
DRAFT 2 April 2004
Page 12 of 16
UO2SO4
20.
K4[Fe(CN)6]
21.
K4[Ni(CN)4]
22.
Na2[Fe(CO)4]
sodium tetracarbonylferrate(2_)
[Co(NH3)6]Cl(SO4) hexaamminecobalt(III) chloride sulfate, or
hexaamminecobalt(3+) chloride sulfate
Fe4[Fe(CN)6]3
iron(III) hexacyanidoferrate(II), or
iron(3+) hexacyanidoferrate(4_)
da
24.
potassium tetracyanidonickelate(0), or
potassium tetracyanidonickelate(4_)
sodium tetracarbonylferrate(_II), or
om
me
n
23.
dioxidouranium(VI) sulfate
potassium hexacyanidoferrate(II), or
potassium hexacyanidoferrate(4_)
tio
n
19.
s
13
Multiple monoatomic constituents vs. homopolyatomic constituents
na
IR-5.4.2.3
lR
ec
Note that oxidation numbers are no longer recommended when naming homopolyatomic
ions. This is to avoid ambiguity. Oxidation numbers refer to the individual atoms of the
element in question, even if they are appended to a name containing a multiplicative prefix, cf.
Example 12 above. To conform to this practice, dimercury(2+) (see Section IR-5.3.2.3)
would have to be named dimercury(I); dioxide(2_) (see Section IR-5.3.3.3) would be
dioxide(_I); and ions such as pentabismuth(4+) (see Section IR-5.3.2.3) and dioxide(1_)
(see Section IR-5.3.3.3), with fractional formal oxidation numbers, could not be named at all.
ov
TlI3
Pr
Examples:
1.
isi
o
Care should be taken to distinguish between multiple monoatomic constituents and
polyatomic constituents. This distinction is often not apparent from the formula, but is tacitly
implied.
Tl(I3)
thallium(3+) iodide
thallium (triiodide), or thallium triiodide(1_),
or thallium(I) (triiodide)
PA
C
2.
thallium tris(iodide), or thallium(III) iodide, or
IU
Both compounds in Examples 1 and 2 have the overall formula TlI3 and both could be named
by the simple stoichiometric name thallium triiodide. However, it is possible _ and usually
desirable _ to convey more information in the name.
_
The compound in Example 1 consists of iodide, I , and thallium, in the proportion 3:1,
_
whereas the compound in Example 2 consists of triiodide(1_), I3 , and thallium in the
proportion 1:1. In the simple stoichiometric name for the first compound, then, the numerical
Page 13 of 16
DRAFT 2 April 2004
14
tio
n
s
prefix 'tris' is used to make it completely clear that three iodide ions are involved rather than
one triiodide ion. The alternative names use the oxidation number III for thallium and the
charge number 3+, respectively, to convey the proportions of the constituents.
Examples:
3.
4.
om
me
n
da
In the first name in Example 2, the parentheses indicate that the electronegative constituent is
a homopolyatomic entity. The second name is even more informative in giving the charge of
the anion, and this can also be indicated indirectly by using the oxidation number for thallium
as shown in the third name.
HgCl2
mercury dichloride, or mercury(II) chloride,
Hg2Cl2
or mercury(2+) chloride
dimercury dichloride, or (dimercury) dichloride,
or dimercury(2+) chloride
Examples:
5.
disodium (trisulfide)
isi
o
Fe2S3
ov
6.
Na2S3
na
lR
ec
In Example 4, the first name is purely stoichiometric, whereas the second name contains more
information in indicating that the compound contains a homodiatomic cation. In the last
name, where the charge of the dication is specified, the prefix 'di' for 'chloride' is not
necessary.
(this indicates the presence of the polyatomic anion),
or sodium trisulfide(2_)
(with the charge on the anion indicated, the numerical prefix
on the cation is not necessary)
diiron tris(sulfide), or iron(III) sulfide
IU
PA
C
Pr
_
Salts which contain anions that are composed of Sn2 chains, as well as those containing
_
several S2 anions, are both referred to as 'polysulfides' but, as demonstrated, names may be
given that provide a distinction between these cases.
Examples:
7.
8.
9.
10.
K 2O
K 2O 2
KO2
KO3
dipotassium oxide
dipotassium (dioxide), or potassium dioxide(2_)
potassium (dioxide), or potassium dioxide(1_)
potassium (trioxide), or potassium trioxide(1_)
DRAFT 2 April 2004
Page 14 of 16
15
12.
BaO2
barium dioxide (simple stoichiometric name), or
barium (dioxide), or barium dioxide(2_) (specifies dianion)
MnO2
manganese dioxide (simple stoichiometric name), or
da
Examples:
11
tio
n
s
Clearly, a simple stoichiometric name like 'potassium dioxide', although strictly speaking
unambiguous (referring to the compound in Example 9), could easily be misinterpreted. In
other cases, based on chemical knowledge, there is no chance of misinterpretation in practice,
and the simple stoichiometric name will most often be used, as in Examples 11 and 12 below.
om
me
n
manganese bis(oxide), or manganese(IV) oxide
(this specifies two oxide ions rather than a dianion)
IR-5.5
NAMES OF ADDITION COMPOUNDS
ec
The term addition compounds covers donor-acceptor complexes and a variety of lattice
compounds. The method described here, however, is relevant not just to such compounds, but
also to multiple salts and to certain compounds of uncertain structure.
isi
o
na
lR
The names of the individual components of such a generalised addition compound are
constructed by using an appropriate nomenclature system, whether compositional,
substitutive or additive. The overall name of the compound is then formed by connecting the
names of the components by 'em' dashes; the proportions of the components are indicated
after the name by a stoichiometric descriptor consisting of arabic numerals separated by a
solidus. The descriptor, in parentheses, is separated from the compound name by a space.
The sequence of names of the individual components is the same as the sequence in the
formula (Section IR-4.4.3.5). In addition compounds, the name of H2O is 'water'. The term
Pr
ov
'hydrate' has a specific meaning, i.e. a compound containing water of crystallization bound in
an unspecified way.
IU
PA
C
Examples:
1.
2.
3.
4.
Page 15 of 16
5.
6.
7.
8.
BF3.2H2O
boron trifluoride—water (1/2)
.
8Kr 46H2O
krypton—water (8/46)
3CdSO4.8H2O
cadmium sulfate—water (3/8)
.
.
Al2(SO4)3 K2SO4 24H2O
CaCl2.8NH3
AlCl3.4EtOH
BiCl3.3PCl5
2Na2CO3.3H2O2
aluminium sulfate—potassium sulfate—water (1/1/24)
calcium chloride—ammonia (1/8)
aluminium chloride—ethanol (1/4)
bismuth(III) chloride—phosphorus(V) chloride (1/3)
sodium carbonate—hydrogen peroxide (2/3)
DRAFT 2 April 2004
16
9.
cobalt(III) oxide—water (1/n)
s
FINAL REMARKS
tio
n
IR-5.6
Co2O3.nH2O
IR-5.7
om
me
n
da
Compositional names are either of the stoichiometric type (which, furthermore, are of the
binary type except in the case of homoatomic species) or of the addition compound type.
Compositional nomenclature is used if little or no structural information is to be conveyed by
the name. However, substitutive or additive nomenclature may be used to indicate the
structure of constituents of a compound that is named overall by compositional
nomenclature. Substitutive nomenclature is described in Chapter IR-6 and additive
nomenclature in Chapters IR-7, IR-8, and IR-9.
REFERENCES
Names for Inorganic Radicals, Pure Appl. Chem., 72, 437 (2000).
IU
PA
C
Pr
ov
isi
o
na
lR
ec
1.
DRAFT 2 April 2004
Page 16 of 16
1
Parent Hydride Names and Substitutive Nomenclature
(Draft March 2004)
s
IR-6
tio
n
CONTENTS
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
IR-6.1 Introduction
IR-6.2 Parent hydrides and unsaturated hydrides
IR-6.2.1 Mononuclear parent hydrides with standard and non-standard bonding
numbers
IR-6.2.2 Homopolynuclear hydrides (other than boron and carbon hydrides)
IR-6.2.2.1 Saturated homonuclear acyclic parent hydrides in which all
atoms have their standard bonding number
IR-6.2.2.2 Homonuclear acyclic parent hydrides with elements
exhibiting non-standard bonding numbers
IR-6.2.2.3 Unsaturated homonuclear acyclic hydrides
IR-6.2.2.4 Homonuclear monocyclic parent hydrides
IR-6.2.2.5 Homonuclear polycyclic parent hydrides
IR-6.2.3 Boron hydrides
IR-6.2.3.1 Stoichiometric names
IR-6.2.3.2 Structural descriptor names
IR-6.2.3.3 Systematic numbering of polyhedral clusters
IR-6.2.3.4 Systematic naming giving hydrogen atom distribution
IR-6.2.4 Heteronuclear hydrides
IR-6.2.4.1 Heteronuclear acyclic parent hydrides in general
IR-6.2.4.2 Hydrides consisting of chains of alternating skeletal atoms
IR-6.2.4.3 Heteronuclear monocyclic parent hydrides; Hantzsch-Widman
nomenclature
IR-6.2.4.4 Skeletal replacement in boron hydrides
IR-6.2.4.5 Heteronuclear polycyclic parent hydrides
IR-6.3 Substitutive names of derivatives of parent hydrides
IR-6.3.1 Use of suffixes and prefixes
IR-6.3.2 Hydrogen substitution in boron hydrides
IR-6.4 Names of ions and radicals derived from parent hydrides
IR-6.4.1 Cations derived from parent hydrides by addition of one or more hydrons
IR-6.4.2 Cations derived from parent hydrides by loss of one or more hydride ions
IR-6.4.3 Substituted cations
IR-6.4.4 Anions derived from parent hydrides by loss of one or more hydrons
IR-6.4.5 Anions derived from parent hydrides by addition of one or more hydride
ions
Page 1 of 34
DRAFT 2 April 2004
2
Substituted anions
Radicals and substituent groups
Substituted radicals or substituent groups
Anionic and cationic centres and radicals in a single molecule or
substituent group
IR-6.5 References
tio
n
da
IR-6.1
s
IR-6.4.6
IR-6.4.7
IR-6.4.8
IR-6.4.9
INTRODUCTION
om
me
n
Substitutive nomenclature is a system in which names are based on names of parent hydrides
implying a defined standard population of hydrogen atoms attached to a skeletal structure.
Names of derivatives of the parent hydrides are formed by citing suffixes or prefixes
appropriate to the substituent groups (or substituents for short) replacing the hydrogen atoms
(preceded by locants when required), joined without a break to the name of the unsubstituted
parent hydride.
na
lR
ec
Substitutive nomenclature is recommended only for derivatives of the parent hydrides named
in Table IR-6.1 (see below), and derivatives of polynuclear hydrides containing only these
elements (see Sections IR-6.2.2 - IR-6.2.4). The bonding numbers of the skeletal atoms are
understood to be as in the Table (these bonding numbers, e.g. 4 for Si and 2 for Se, are
termed standard bonding numbers). Other bonding numbers must be indicated by an
appropriate designator (the 'λ convention', see Section IR-6.2.2.2 and Section P-14.1.3 of
isi
o
Ref. 1).
ov
In general, relevant practices and conventions of substitutive nomenclature as applied to
organic compounds1 are also followed here.
IU
PA
C
Pr
Constructing a substitutive name involves the replacement of hydrogen atoms in a parent
structure with other atoms or atom groups. Related operations, often considered to be part of
substitutive nomenclature, are skeletal replacement (Section IR-6.2.4.1) and functional
replacement in oxoacid parents (Section IR-8.6). In most cases, the compounds named
substitutively in the present chapter may alternatively and equally systematically be named
additively (Chapter IR-7), but it is important to note that for the parent hydrides presented
here such additive names cannot be used as parent names in substitutive nomenclature.
Neutral boron hydrides are called boranes. The basic aspects of borane nomenclature are
provided in Section IR-6.2.3; more advanced aspects will be treated in a future IUPAC
publication.
DRAFT 2 April 2004
Page 2 of 34
3
PARENT HYDRIDE NAMES
IR-6.2.1
Mononuclear parent hydrides with standard and non-standard bonding numbers
tio
n
s
IR-6.2
om
me
n
Table IR-6.1 Parent names of mononuclear hydrides
da
The mononuclear hydrides of elements of groups 13-17 of the Periodic Table play a central
role in substitutive nomenclature. They are used as parent hydrides as indicated above with
the parent names given in Table IR-6.1.
(Insert separate landscape Table)
λ5-phosphane
λ1-phosphane
λ6-sulfane
λ2-stannane
lR
PH5
PH
SH6
SnH2
na
Examples:
1.
2.
3.
4.
ec
In cases where the bonding number deviates from the standard number defined above, it must
be indicated in the hydride name by means of an appropriate superscript appended to the
Greek letter λ, this symbol being separated from the name in Table IR-6.1 by a hyphen.
Homopolynuclear parent hydrides (other than boron and carbon hydrides)
IR-6.2.2.1
Homonuclear acyclic parent hydrides in which all atoms have their standard
bonding number
ov
isi
o
IR-6.2.2
IU
PA
C
Pr
Names are constructed by prefixing the 'ane' name of the corresponding mononuclear
hydride from Table IR-6.1 with the appropriate multiplicative prefix ('di', 'tri', 'tetra', etc. see
Table IV∗) corresponding to the number of atoms of the chain bonded in series.
∗
Examples:
1.
2.
3.
4.
5.
6.
HOOH
H2NNH2
H2PPH2
H3SnSnH3
dioxidane, or hydrogen peroxide
diazane, or hydrazine
diphosphane
distannane
HSeSeSeH
SiH3SiH2SiH2SiH3
triselane
tetrasilane
Tables numbered with a Roman numeral are collected together at the end of this book.
Page 3 of 34
DRAFT 2 April 2004
4
tio
n
s
The compositional name 'hydrogen peroxide' (cf. Chapter IR-5) is an alternative to 'dioxidane'
for H2O2 itself, but is not applicable as a parent hydride name in substitutive nomenclature.
In Section P-68.3.1.2.1 of Ref. 1 organic derivatives of H2NNH2 are named on the basis of
Homonuclear acyclic parent hydrides with elements exhibiting non-standard bonding
numbers
om
me
n
IR-6.2.2.2
da
'hydrazine' as a parent name.
In cases where the skeletal atoms of a hydride chain are the same but one or more has a
bonding number different from the standard values defined by Table IR-6.1, the name of the
hydride is formed as if all the atoms showed standard bonding numbers, but is preceded by
locants, one for each non-standard atom, each locant qualified without a space by λn, where n
is the appropriate bonding number.
isi
o
Examples:
1.
na
lR
ec
When a choice is needed between the same skeletal atom in different valence states, the one in
a non-standard valence state is preferred for assignment of the lower locant. If a further
choice is needed between the same skeletal atom in two or more non-standard valence states,
preference for the lower locant or locants is given in order of decreasing numerical value of
the bonding number, i.e. λ6 is preferred to λ4.
1 23
4
H5SSSH4SH
Pr
2.
ov
1λ6, 3λ6-tetrasulfane (not 2λ6, 4λ6)
12
3
4
5
HSSH4SH4SH2 SH
PA
C
2λ6, 3λ6, 4λ4-pentasulfane (not 2λ4, 3λ6, 4λ6)
IU
3.
H4PPH3PH3PH4
lλ5, 2λ5, 3λ5, 4λ5-tetraphosphane
4.
HPbPbPbH
1λ2, 2λ2, 3λ2 -triplumbane
DRAFT 2 April 2004
Page 4 of 34
5
Unsaturated homonuclear acyclic hydrides
s
IR-6.2.2.3
HN=NH
HSb=SbH
1 2
3.
3
diazene
distibene
4 5
H2NN=NHNNH2
ec
Examples:
1.
2.
om
me
n
da
tio
n
Chains containing unsaturation are accommodated in substitutive nomenclature by the
methods used with alkenes and alkynes (see Section P-31.1 of Ref. 1), i.e. the name of the
corresponding saturated chain hydride is modified by replacing the 'ane' suffix with 'ene' in
the case of a double bond and 'yne' in the case of a triple bond. If there is one of each, the
suffix becomes 'en' ...'yne' with appropriate locants; 'diene' is used when there are two double
bonds, and so on. In each case the position(s) of unsaturation is/are indicated by (a)
numerical locant(s) immediately preceding the suffix(es). Locants are chosen to be as low as
possible.
pentaaz-2-ene (not pentaaz-3-ene,
lR
and not pentaz-2-ene)
Homonuclear monocyclic parent hydrides
ov
IR-6.2.2.4
isi
o
na
Unsaturated acyclic hydrides are not classified as parent hydrides. Because of the hierarchical
rules of substitutive nomenclature, the numbering of the double and triple bonds may not be
fixed until various groups and modifications with higher priority have been numbered. (See
Section IR-6.4.9 for an example).
There are three main ways of giving parent names to homonuclear monocyclic hydrides:
Pr
(i) by using the Hantzsch-Widman (H-W) name (see Section IR-6.2.4.3 and Section P22.2.2 of Ref. 1);
IU
PA
C
(ii) by using the relevant replacement prefix ('a' term) from Table X together with the
appropriate multiplicative prefix to indicate replacement of carbon atoms in the corresponding
carbocyclic compound;
Page 5 of 34
(iii) by adding the prefix 'cyclo' to the name of the corresponding unbranched,
unsubstituted chain (see Sections IR-6.2.2.1 - IR-6.2.2.3 and Section P-22.2.4 of Ref. 1).
Each method is used in Examples 1-4 below. When naming organic derivatives of noncarbon homonuclear monocyclic parent hydrides, the Hantzsch-Widman name is preferred
DRAFT 2 April 2004
6
s
for rings with 3 to 10 members. For larger rings, the names given by the second method are
preferred.
NH
HN
NH
om
me
n
N
H
da
HN
tio
n
Examples:
1.
(i) H-W name: pentazolidine
(ii) pentaazacyclopentane
(iii) cyclopentaazane
2.
H2
Si
ec
H2
Si
SiH2
H2Si
SiH2
lR
H2Si
Si
H2
IU
PA
C
Pr
ov
3.
isi
o
na
Si
H2
(i) H-W name: octasilocane
(ii) octasilacyclooctane
(iii) cyclooctasilane
1
H2
Ge
HGe
3
GeH
2
(i) H-W name: 1H-trigermirene
(ii) trigermacyclopropene
(iii) cyclotrigermene
4.
DRAFT 2 April 2004
Page 6 of 34
7
2
3
N
N
1
s
N4
N
5
1
2
N
N
N
om
me
n
HN
da
(i) H-W name: 1H-pentazole
tio
n
HN
3
N
4
(ii) pentaazacyclopenta-1,3-diene
(iii) cyclopentaaza-1,3-diene
IR-6.2.2.5
lR
ec
Note that in Example 4 the numbering for the H-W name differs from that for the other two
methods; H-W priorities depend on the H-atom position, and those in (ii) and (iii) on the
locations of the double bonds.
Homonuclear polycyclic parent hydrides
na
Parent names of homonuclear polycycles may be constructed by any one of three methods:
isi
o
(i) by specifying the fusion of relevant monocycles (Section P-25.3.2 of Ref. 1), each named
by the Hantzsch-Widman system (see Section IR-6.2.4.3);
Pr
ov
(ii) using a skeletal replacement prefix ('a' term) from Table X together with the appropriate
multiplicative prefix to indicate replacement of the carbon atoms in the corresponding
carbocyclic compound;
PA
C
(iii) specifying the ring structure using the von Baeyer notation (Section P-23.4 of Ref. 1) in
combination with the name of the corresponding linear hydride as derived in Section IR6.2.2.1.
IU
Examples:
1.
HSi 7
HSi 6
8
5
Si
H
Page 7 of 34
H
Si
H
Si
Si
1
8a
2
Si 4a 4
Si
H
3
DRAFT 2 April 2004
SiH
SiH
8
2.
1
H2
Si
H2Si 9 10
SiH
2
H2Si 8
SiH
5
Si
H2
6
SiH2
4
SiH2
Si
H2
om
me
n
7
3
da
H2
Si
tio
n
s
(i) hexasilinohexasiline
(ii) decasilanaphthalene
IR-6.2.3
Boron hydrides
IR-6.2.3.1
Stoichiometric names
ec
(iii) bicyclo[4.4.0]decasilane
(von Baeyer name)
lR
Neutral polyboron hydrides are called boranes and the simplest possible parent structure,
BH3, is given the name 'borane'. The number of boron atoms in a boron hydride molecule is
ov
diborane(6)
icosaborane(16)
Structural descriptor names
PA
C
IR-6.2.3.2
B 2H 6
B20H16
Pr
Examples:
1.
2.
isi
o
na
indicated by a numerical prefix. The principal difference between this system of naming and
hydrocarbon nomenclature is that the number of hydrogen atoms must be defined; it cannot
be inferred from simple bonding considerations. The number of hydrogen atoms is indicated
by the appropriate arabic numeral in parentheses directly following the name. Such names
convey only compositional information.
IU
More structural information is obtained by augmenting the stoichiometric name by a
structural descriptor. The descriptor is based on electron-counting relationships4 and is
presented in Table IR-6.2.
Table IR-6.2. Summary of common polyboron hydride structure types according to
stoichiometry and electron-counting relationships.
DRAFT 2 April 2004
Page 8 of 34
9
Parent
hydride
Description of structure
closo
n+1
BnHn+2
Closed polyhedral structure with triangular faces only.
nido
n+2
BnHn+4
Nest-like non-closed polyhedral structure; n vertices of
the parent (n+1)-atom closo polyhedron occupied.
arachno
n+3
BnHn+6
Web-like non-closed polyhedral structure; n vertices of
the parent (n+2)-atom closo polyhedron occupied.
hypho
n+4
BnHn+8
Net-like non-closed polyhedral structure; n vertices of
the parent (n+3)-atom closo polyhedron occupied.
klado
n+5
BnHn+10 Open branch-like polyhedral structure; n vertices of the
parent (n+4)-atom closo polyhedron occupied.
ec
om
me
n
da
tio
n
s
Descriptor Skeletal
electron
pairs
lR
(The structural relationships are often represented by a Rudolph diagram.5)
na
Examples:
1.
isi
o
1
ov
Pr
PA
C
2
5
4
3
nido-pentaborane(9), B5H9
IU
2.
Page 9 of 34
1
2
4
3
DRAFT 2 April 2004
10
arachno-tetraborane(10), B4H10
as follows:
tio
n
s
The two structures in Examples 1 and 2 can be thought of as related to that of closo-B6H62-
1
1
2
-BH, -2e+4H
3
4
6
2
3
om
me
n
4
5
da
5
-BH, +2H
1
2
ec
4
lR
3
isi
o
na
The structures are obtained formally by removal of one (Example 1) or two (Example 2) BH
groups from, and the addition of the appropriate number of electrons and hydrogen atoms to,
the highest connectivity vertices of closo-B6H62-.
ov
It should be noted that the prefixes nido, arachno, etc. are not used for the simplest boranes
for which formal derivation from closo parent structures by successive subtractions might
seem to be far-fetched.
Pr
Chain compounds may be explicitly specified as such by using the prefix 'catena'.
IU
PA
C
Examples:
3.
diborane(6), B2H6
DRAFT 2 April 2004
Page 10 of 34
11
s
H2BBHBH2 catena-triborane(5)
HB=BBH2
catena-triborene(3)
tio
n
4.
5.
6.
H
H
om
me
n
H
da
For cyclic systems, the prefix 'cyclo' in connection with the name of the corresponding chain
compound or the Hantzsch-Widman (H-W) nomenclature system (cf. Section IR-6.2.4.3)
may be used.
B
B
B
B
H
Systematic numbering of polyhedral clusters
lR
IR-6.2.3.3
ec
cyclotetraborane
H-W name: tetraboretane
isi
o
na
It is necessary to number the boron skeleton for each cluster systematically, so as to permit
the unambiguous naming of the substituted derivatives. For this purpose, the boron atoms of
closo structures are considered as occupying planes disposed sequentially, perpendicular to
the axis of highest order symmetry. (If there are two such axes, the 'longer', in terms of the
greater number of perpendicular planes crossed, is chosen).
PA
C
Pr
ov
Numbering begins at the nearest boron atom when the cluster is viewed along this axis and
proceeds either clockwise or anti-clockwise, dealing with all skeletal atoms of the first plane.
Numbering then continues in the same sense in the next plane, beginning with the boron atom
nearest to the lowest numbered boron atom in the preceding plane when going forward in the
direction of numbering.
IU
Example:
1.
Page 11 of 34
DRAFT 2 April 2004
12
4
1
5
7
tio
n
s
8
3
9
2
10
da
6
om
me
n
closo-B10H102- (hydrogen atoms omitted for clarity)
lR
ec
The numbering in nido clusters is derived from that of the related closo cluster. In the case of
arachno and more open clusters, the opened side is presented towards the observer and the
boron atoms considered as projected onto a plane at the rear. They are then numbered
sequentially in zones, commencing at the central boron atom of highest connectivity and
proceeding clockwise or anti-clockwise until the innermost zone is completed. The next zone
is then numbered in the same sense starting from the 12 o'clock position, and so on until the
outermost zone is completed. This treatment means that the numbering of the closo parent is
unlikely to carry over into the corresponding arachno system.
na
Examples:
2.
4
5
6
1
2
7
Pr
ov
isi
o
3
PA
C
arachno-B7H13 (hydrogen atoms omitted for clarity)
IU
When there is a choice, the molecule is so oriented that the 12 o'clock position is decided by
sequential application of the following criteria:
(i) the 12 o'clock position lies in a symmetry plane, which contains as few boron atoms as
possible;
(ii) the 12 o'clock position lies in that portion of the symmetry plane which contains the
greatest number of skeletal atoms;
DRAFT 2 April 2004
Page 12 of 34
13
(iii) the 12 o'clock position lies opposite the greater number of bridging atoms.
Systematic naming giving hydrogen atom distribution
da
IR-6.2.3.4
tio
n
s
The use of criteria (i)-(iii) may fail to effect a decision, and where a symmetry plane is lacking
they are inapplicable. In such cases the general principles of organic numbering are used,
such as choosing a numbering scheme which gives substituted atoms the lowest locants.
om
me
n
In open boranes each boron atom can be assumed to carry at least one terminal hydrogen
atom. However, it is necessary to specify the positions of the bridging hydrogen atoms by
using the symbol µ, preceded by the locants for the skeletal positions so bridged in ascending
numerical order. The designator H is used for the bridging hydrogen atoms in the name.
isi
o
na
lR
ec
Example:
1.
2,3:2,5:3,4:4,5-tetra-µH-nido-pentaborane(9)
Heteronuclear parent hydrides
PA
C
IR-6.2.4
Pr
ov
This method of locating bridging hydrogen atoms is adapted from the 'indicated hydrogen'
method in organic nomenclature (see Section P-14.6 of Ref. 1). The 'indicated hydrogen'
method would yield the name (2,3-µH),(2,5-µH),(3,4-µH),(4,5-µH)-nidopentaborane(9).
IU
IR-6.2.4.1
Page 13 of 34
Heteronuclear acyclic parent hydrides in general
When at least four carbon atoms in an unbranched-chain parent hydrocarbon are replaced by
heteroatoms, alike or different, and the terminal carbon atoms either remain or are replaced by
P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, or Tl, skeletal replacement nomenclature ('a'
nomenclature) may be used to indicate the heteroatoms (see Section P-15.4 of Ref. 1).
DRAFT 2 April 2004
14
da
tio
n
s
In this method, the chain is named first as if it were composed entirely of carbon atoms. Any
heteroatoms in the chain are then designated by appropriate replacement prefixes ('a' terms)
from Table X cited in the priority order given by Table VI, each preceded by its appropriate
locant. The locants are assigned by numbering the chain from that end which gives lower
locants to the heteroatom set as a whole and, if these are equal, from that end which gives the
lower locant or locant set to the replacement prefix first cited. If there is still a choice, lower
locants are assigned to the sites of unsaturation.
ec
om
me
n
Only chains with four or more heteroatoms (or strictly speaking, four or more heterounits)
are given parent names constructed in this way. A heterounit is a sequence of heteroatoms
which is in itself the skeleton of a parent hydride, e.g. SS and SiOSi (cf. Section IR-6.2.4.2)
but not OSiO. Heteroatoms must not belong to the principal characteristic group (see Section
IR-6.3.1) (if there is one) when counting them for this purpose. Heteronuclear chains with
fewer heterounits, and heteronuclear chains not terminating in any of the atoms listed above,
are named substitutively as derivatives of homonuclear parent hydrides and are not
themselves used as parents.
lR
Examples:
1.
NH2
na
H 2N
H
N
Pr
ov
2.
isi
o
N-(2-aminoethyl)ethane-1,2-diamine
H
N
H 2N
NH2
N
H
N,N'-bis(2-aminoethyl)ethane-1,2-diamine
IU
PA
C
3.
11 10 9
8
7 6
5
4
3
21
CH3OCH2CH2OCH2CH2SiH2CH2SCH3
7,10-dioxa-2-thia-4-silaundecane
(Parent name. Note the name is not 2,5-dioxa-10-thia-8-silaundecane because the
locant set 2,4,7,10 takes precedence over the locant set 2,5,8,10)
DRAFT 2 April 2004
Page 14 of 34
15
tio
n
s
Unambiguous parent names for non-carbon-containing heteronuclear chains can be derived
from a hydrocarbon parent or a non-carbon homonuclear chain parent (cf. Section IR6.2.2.1). Alternatively, heteronuclear chains may be named additively by the method
described in Section IR-7.4. However, such names cannot be used as parent names in
substitutive nomenclature.
da
Example:
4.
om
me
n
SiH3SiH2SiH2GeH2SiH3
1,2,3,5-tetrasila-4-germapentane (not 1,3,4,5-tetrasila-2-germapentane),
or 2-germapentasilane, or
1,1,1,2,2,3,3,4,4,5,5,5-dodecahydrido-2-germy-1,2,3,5-tetrasily-[5]catena
Hydrides consisting of chains of alternating skeletal atoms
ec
IR-6.2.4.2
lR
Chains hydrides with a backbone of alternating atoms of two elements A and E, neither of
which is carbon, i.e. of sequences (AE)nA, where element A occurs later in the sequence of
Table VI, can be named by successive citation of the following name parts:
na
(i) a numerical prefix (Table IV) denoting the number of atoms of element A, with no elision
of a terminal vowel of this prefix;
isi
o
(ii) replacement prefixes ending in 'a' (Table X) denoting elements A and E in that order (with
elision of the terminal 'a' of the replacement prefix before another 'a' or an 'o');
SnH3OSnH2OSnH2OSnH3
SiH3SSiH2SSiH2SSiH3
PH2NHPHNHPH2
SiH3NHSiH3
PA
C
Pr
Examples:
1.
2.
3.
4.
ov
(iii) the ending 'ne'.
IU
5.
IR-6.2.4.3
Page 15 of 34
1
2 34
5
6
tetrastannoxane
tetrasilathiane
triphosphazane
disilazane
7
PH2N=PNHPHNHPH2
tetraphosphaz-2-ene
The first four structures are parent hydrides, but not the unsaturated compound (cf. remarks
in Section IR-6.2.2.3).
Heteronuclear monocyclic parent hydrides; Hantzsch-Widman nomenclature
DRAFT 2 April 2004
16
tio
n
s
For heteronuclear monocyclic parent hydrides there are two general naming systems and, in certain cases, a
third possibility.
om
me
n
da
(i) In the (extended) Hantzsch-Widman (H-W) system (Section P-22.2.2 of Ref. 1), names
are constructed so as to convey the ring size, the presence of heteroatoms (i.e. non-carbon
atoms) and the degree of hydrogenation (either mancude, i.e. with the maximum number of
non-cumulative double bonds, or saturated) by means of characteristic endings. These
endings are given in Table IR-6.3. (Hydrides with intermediate degrees of hydrogenation are
named by the use of the prefix 'hydro' together with an appropriate multiplicative prefix.
However, such hydrides are not parents.)
lR
ec
The order of citation of the heteroatoms follows Table VI, i.e. F > Cl > Br > I > O >….etc.,
where '>' means 'is cited before'. Locants are assigned to the heteroatoms so as to ensure first
that the locant '1' is given to the atom cited first and then that the total set of locants is as low
as possible consistent with sequential numbering of the ring positions (ordering locant sets
lexicographically). The heteroatoms are cited by the replacement prefixes ('a' terms) given in
Table X together with appropriate multiplicative prefixes. In the case of six-membered rings,
the ring heteroatom which is cited last decides which of the alternative endings in Table IR6.3 is chosen.
isi
o
na
Tautomers may be distinguished using indicated hydrogen to specify the location of the
hydrogen atom(s) which can be placed in several ways [and thus, indirectly, the location of
the double bond(s)], as in Example 2 below.
ov
Table IR-6.3 Endings in the Hantzsch-Widman system
IU
Saturated
irane ('iridine' for rings containing N)
4
5
irene ('irine' for rings
with N as only heteroatom)
ete
ole
6(A)b
ine
ane
6(B)b
ine
inane
6(C)b
inine
inane
7
epine
epane
PA
C
3
Pr
Number of
Mancudea
atoms in ring
etane ('etidine' for rings containing N)
olane ('olidine' for rings containing N)
DRAFT 2 April 2004
Page 16 of 34
17
ocane
onane
ecane
s
ocine
onine
ecine
tio
n
8
9
10
a Maximum number of non-cumulative double bonds.
da
b 6(A) is used when the last-cited heteroatom is O, S, Se, Te, Po, or Bi; 6(B) is used when
om
me
n
the last-cited heteroatom is N, Si, Ge, Sn, or Pb; and 6(C) is used when the last-cited
heteroatom is F, Cl, Br, I, P, As, Sb, B, Al, Ga, In, or Tl.
(ii) Alternatively, the name is based on the name of the corresponding carbocycle, and the
heteroatoms are indicated by the replacement prefixes ('a' terms) from Table X together with
appropriate multiplicative prefixes. The order of citation is again given by Table VI.
lR
ec
(iii) For the special case of rings of two alternating skeletal atoms (as in Examples 3-8
below), the name is constructed using the prefix 'cyclo' followed by the replacement prefixes
(Table X) cited in the reverse of the order in which the corresponding elements appear in
Table VI. The name ends with 'ane' if the repeating unit is saturated.
IU
PA
C
ov
Pr
Examples:
1.
isi
o
na
Method (ii) is only recommended for saturated rings and mancude rings (rings with the
maximum number of non-cumulative double bonds) and method (iii) only for saturated rings.
The Hantzsch-Widman names are preferred for rings with up to 10 members and method (ii)
is preferred for saturated rings with more than 10 members. For more rules on large rings,
see Section P-22.2.3 of Ref. 1.
Page 17 of 34
H2
Ge
H2Si
SiH2
(i) H-W name: disilagermirane
(ii) disilagermacyclopropane
2.
DRAFT 2 April 2004
18
HSi
3
SiH
2
H
Ge
HSi
1
SiH2
2
(a)
1
(b)
s
H2
Ge
tio
n
3
da
H-W names: 3H-1,2,3-disilagermirene (a), and 1H-1,2,3- disilagermirene (b)
3.
om
me
n
1
O
4
HSb
2
SbH
O
3
lR
ec
(i) H-W name: 1,3,2,4-dioxadistibetane
(ii) 1,3-dioxa-2,4-distibacyclobutane
(iii) cyclodistiboxane
6
H
N
2
HB
1
BH
HN
4
NH
5
B
H
3
(i) H-W name: 1,3,5,2,4,6-triazatriborinane
(ii) 1,3,5-triaza-2,4,6-triboracyclohexane
(iii) cyclotriborazane
Pr
ov
isi
o
na
4.
IU
PA
C
5.
1
6
O
HB
O
5
2
BH
4
B
H
O
3
(i) H-W name: 1,3,5,2,4,6-trioxatriborinane
(ii) 1,3,5-trioxa-2,4,6-triboracyclohexane
(iii) cyclotriboroxane
DRAFT 2 April 2004
Page 18 of 34
19
6.
1
S
BH
4
B
H
S
3
da
5
2
s
S
tio
n
6
HB
om
me
n
(i) H-W name: 1,3,5,2,4,6-trithiatriborinane
(ii) 1,3,5-trithia-2,4,6-triboracyclohexane
(iii) cyclotriborathiane
ec
The names borazole, boroxole and borthiole, respectively, for the three compounds in
Examples 4, 5 and 6 have been abandoned long ago as they imply five-membered rings in the
Hantzsch-Widman system. The names borazin(e), boroxin and borthiin indicate sixmembered rings with unsaturation and only one boron atom and one other heteroatom
(although the order of the element name stems is wrong) and are also not recommended.
6
1
N
HSi
2
SiH
N
4
N
5
Si
H
3
ov
isi
o
na
lR
Example:
7.
Pr
(i) H-W name: 1,3,5,2,4,6-triazatrisiline
(ii) 1,3,5-triaza-2,4,6-trisilacyclohexa-1,3,5-triene
PA
C
Where ring atoms have a connectivity different from their standard bonding number (see
Section IR-6.2.1), their actual bonding number is expressed as an arabic superscript to the
Greek letter lambda following immediately after an appropriate locant.
IU
Example:
8.
Page 19 of 34
DRAFT 2 April 2004
20
1
N
N
5
PH2
4
P
H2
s
H 2P
2
N
tio
n
6
3
IR-6.2.4.4
Skeletal replacement in boron hydrides
om
me
n
da
(i) H-W name: 1,3,5,2λ5, 4λ5, 6λ5-triazatriphosphinine
(ii) 1,3,5-triaza-2λ5, 4λ5, 6λ5-triphosphacyclohexa-1,3,5-triene
ec
It is possible that the essential skeletal structure of the boron hydrides is preserved in
derivatives in which one or more of the boron atoms are replaced by other atoms. The names
of such species are formed by an adaptation of replacement nomenclature, giving
carbaboranes, azaboranes, phosphaboranes, thiaboranes, etc.
ov
isi
o
na
lR
In the heteroboranes, the number of nearest neighbours to the heteroatom is variable and can
be 5, 6, 7, etc. Therefore, in the adaptation of replacement nomenclature to polyborane
compounds, the replacement of a boron atom by another atom is indicated in the name along
with the number of hydrogen atoms in the resulting polyhedral structure. The prefixes closo,
nido, arachno, etc., are retained as described for boron hydrides (Section IR-6.2.3.2). The
positions of the supplanting heteroatoms in the polyhedral framework are indicated by
locants which are the lowest possible numbers taken as a set consistent with the numbering of
the parent polyborane. If a choice remains for locant assignment within a given set, then
priority for low numbering should be assigned to the element encountered first using Table
VI.
PA
C
Pr
The hydrogen atom population of the actual compound concerned (and not that of the parent
all-boron skeletal compound) is added as an arabic numeral in parentheses at the end of the
name. The numeral is retained in hydrogen substitution.
B10C2H12
B3C2H5
dicarba-closo-dodecaborane(12)
1,5-dicarba-closo-pentaborane(5)
IU
Examples:
1.
2.
DRAFT 2 April 2004
Page 20 of 34
21
3
tio
n
s
1
4
da
2
3.
om
me
n
5
B4C2H8
4,5:5,6-di-µH-2,3-dicarba-nido-hexaborane(8)
2
ec
1
na
lR
6
4
isi
o
3
5
IU
PA
C
Pr
Examples:
4.
ov
Note that locants for skeletal replacement take precedence over those for bridging hydrogen
atoms. The number of bridging hydrogen atoms is usually different for heteroboranes
compared with parent polyboranes, and for numbering purposes only the symmetry of the
parent boron skeleton is considered.
Page 21 of 34
DRAFT 2 April 2004
10
6
5
9
8
7
1
2
da
4
tio
n
s
22
om
me
n
3
= Co
6,9-bis(η5-pentamethylcyclopentadienyl)-5,6:6,7:8,9:9,10-tetra-µH-6,9-dicobaltanido-decaborane(12) (one terminal hydrogen on each boron atom omitted for clarity).
5.
lR
ec
1
3
2
4
CO
na
5
isi
o
CO
CO
6
= Fe
Heteronuclear polycyclic parent hydrides
Pr
IR-6.2.4.5
ov
2,2,2-tricarbonyl-1,6-dicarba-2-ferra-closo-hexaborane(5)
PA
C
Parent names of heteronuclear polycycles may be constructed by three methods:
IU
(i) specifying the fusion of relevant monocycles (see Section P-25.3.2 of Ref. 1), named by
the Hantzsch-Widman system (see Section IR-6.2.4.3);
(ii) using replacement prefixes ('a' terms) from Table X to specify replacement of carbon
atoms in the corresponding carbocyclic compound. Heteroatoms are cited in the order given
by Table VI and appropriate multiplicative prefixes are added;
DRAFT 2 April 2004
Page 22 of 34
23
tio
n
s
(iii) for ring systems consisting of repeating units, using the von Baeyer notation to specify
the ring structure combined with the appropriate multiplicative prefix and the replacement
prefixes from Table X appropriate to the repeating unit, cf. the names discussed in Section
IR-6.2.4.2.
H
N
HB
8
HN
5
6
B
H
8a
H
N
2
B
1
BH
om
me
n
7
da
Example:
1.
N
4a
4
B
H
NH
3
{Numbering is only for method (ii)}
lR
ec
(i): octahydro[1,3,5,2,4,6]triazatriborinino[1,3,5,2,4,6]triazatriborinine
(ii): octahydro-1,3,4a,6,8-pentaaza-2,4,5,7,8a-pentaboranaphthalene
(iii): bicyclo[4.4.0]pentaborazane
isi
o
na
In this example, names (i) and (ii) need the additional 'octahydro' prefixes because the
available parent hydrides for these constructions (triazatriborinine and naphthalene,
respectively) are mancude (i.e. have the maximum number of non-cumulative double bonds).
SUBSTITUTIVE NAMES OF DERIVATIVES OF PARENT HYDRIDES
IR-6.3.1
Use of suffixes and prefixes
ov
IR-6.3
IU
PA
C
Pr
Substituent groups (or substituents), considered as replacing hydrogen atoms in parent
hydrides, are named using appropriate suffixes ('ol', 'thiol', 'peroxol', 'carboxylic acid', etc.)
and prefixes ('hydroxy', 'phosphanyl', 'bromo', 'nitro', etc.). Substituent suffixes are ranked,
and suffixes and prefixes extensively listed, in Appendices 1 and 2 of Ref. 1. The case of
substituents formed by removal of one or more hydrogen atoms from a parent hydride is
explained briefly, with examples, in Section IR-6.4.7, and suffixes and prefixes for many
common inorganic substituents are included in Tables III and IX, respectively.
Page 23 of 34
Some substituents are always cited as prefixes, most notably halogen atoms. Otherwise, the
highest-ranking substituent (the principal characteristic group) is cited as a suffix and the rest
of the substituents as prefixes. Except for 'hydro', prefixes are cited in alphabetical order
before the name of the parent hydride, parentheses being used to avoid ambiguity.
DRAFT 2 April 2004
24
da
tio
n
s
Multiplicative prefixes indicate the presence of two or more identical substituents; if the
substituents themselves are substituted, the prefixes 'bis', 'tris', 'tetrakis', etc. are used. In the
case of a multiplicative prefix ending in 'a' and a suffix starting with a vowel, the 'a' is elided
(see Example 2 below). The final 'e' of a parent hydride name is elided in front of a suffix
starting with a vowel (see Examples 1 and 6 below).
om
me
n
Where there is a choice of parent hydride among those listed in Table IR-6.1 (or
corresponding hydrides with non-standard bonding numbers, cf. Section IR-6.2.2.2), the
name is based on the parent hydride of the element occurring first in the sequence: N, P, As,
Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, Tl, O, S, Se, Te, C, F, Cl, Br, I.
lR
ec
The above exposition is only a very brief overview of the most important principles of
substitutive nomenclature. In Ref. 1, an extensive system of rules is developed for choosing
one name among the many unambiguous substitutive names that may often be constructed
for organic compounds. A corresponding extensive set of rules has not been developed for
non carbon-containing compounds, partly because many such compounds can just as well be
given additive names (Chapter IR-7), and often are.
Examples:
1.
2.
isi
o
SF6
TlH2CN
or hexafluoridosulfur (additive)
thallanecarbonitrile (substitutive),
SiH3NH2
or cyanidodihydridothallium (additive)
silanamine (substitutive),
PH2Cl
PH2Et
TlH2OOOTlH2
PbEt4
or amidotrihydridosilicon (additive)
chlorophosphane
ethylphosphane
trioxidanediylbis(thallane)
tetraethylplumbane (substitutive),
GeH(SMe)3
PhGe(Cl)2(SiCl3)
or tetraethyllead (additive)
tris(methylsulfanyl)germane
trichloro[dichloro(phenyl)germyl]silane,
Pr
4.
IU
PA
C
5.
6.
7.
8.
9.
10.
11.
silanol
sulfanediol (substitutive),
or dihydroxidosulfur (additive)
hexafluoro-λ6-sulfane (substitutive),
ov
3.
SiH3OH
S(OH)2
na
The following names exemplify the above principles. In some cases, additive names are given
for comparison.
DRAFT 2 April 2004
Page 24 of 34
25
MePHSiH3
tio
n
not (methylphosphanyl)silane or
(silylphosphanyl)methane
s
12.
not dichloro(phenyl)(trichlorosilyl)germane
methyl(silyl)phosphane,
4,4,4-tribromo-2λ2-tetragermane
(numbering of parent fixed by λ designator)
14.
32
ec
H3GeGeGeH2GeBr3
1
lR
Examples:
13.
om
me
n
da
For polynuclear parent hydrides, numerical locants are often needed to specify the positions
of substituent groups. If there are several equivalent numberings of the parent hydride
skeletal atoms relative to the substituents after relevant rules from Section IR-6.2 have been
applied, the numbering is chosen which leads to the lowest set of locants for the compound as
a whole. If there is still a choice, lowest locants are assigned to the substituent cited first in the
name. If no ambiguity arises, some or all locants can be omitted, as in Examples 21 and 22
below. In Ref. 1, preferred names always contain all locants, except if the only locant is the
number '1'.
15.
3
2
na
H3SiSiH2SiH2COOH
1
isi
o
MeNHN=NH
16.
1
trisilane-1-carboxylic acid
2
3
4
3-methyltriaz-1-ene (not 1-methyltriaz-3-ene)
(numbering of parent fixed by position of unsaturation)
5
ov
ClSiH2SiHClSiH2SiH2SiH2Cl
Pr
17.
1,2,5-trichloropentasilane (not 1,4,5-)
3
2
1
PA
C
C3H7SnH2SnCl2SnH2Br
IU
18.
Page 25 of 34
1-bromo-2,2-dichloro-3-propyltristannane
(1-bromo preferred to 3-bromo)
1
2 3
4 5
6 7
HSnCl2OSnH2OSnH2OSnH2Cl
1,1,7-trichlorotetrastannoxane
19.
DRAFT 2 April 2004
26
H
N
1
4
SiHEt
3
s
2
Me2Si
tio
n
N
H
20.
om
me
n
da
H-W name: 4-ethyl-2,2-dimethyl-1,3,2,4-diazadisiletane
4-ethyl-2,2-dimethylcyclodisilazane
(locant set 2,2,4 preferred to 2,4,4 in both names)
Et3PbPbEt3
1,1,1,2,2,2-hexaethyldiplumbane,
or hexaethyldiplumbane (substitutive),
or bis(triethyllead)(Pb−Pb) (additive)
MeNHN=NMe
1,3-dimethyltriaz-1-ene,
or 1,3-dimethyltriazene
ec
21.
na
lR
The names of branched structures are based on the longest available unbranched chain, which
is regarded as defining the parent hydride, and the names of the shorter chains, which are
treated as substituents and appropriately cited. Once the longest chain has been chosen, it is
numbered so as to give the lowest set of locants to the substituents.
isi
o
Examples:
22.
B
BH2
H 2B
ov
Pr
H 2B
2-boranyltriborane(5)
IU
PA
C
23.
1
2
5
H3SiH2Si
7
SiH2SiH2SiH3
3
HSi
6
4
SiH
H3Si
SiH2SiH3
4-disilanyl-3-silylheptasilane
(not 4-disilanyl-5-silylheptasilane)
DRAFT 2 April 2004
Page 26 of 34
27
s
If a choice of principal chain cannot be made on the basis of chain length alone, unsaturation
is the next selection criterion and then the greatest number of substituents.
ClH2Si
tio
n
Example:
24.
SiH2Cl
1
H
Si
H
4
SiH
5
SiHCl2
om
me
n
Cl3Si
da
2 3
HSi
1,1,1,5,5-pentachloro-2,4-bis(chlorosilyl)pentasilane
(all other 5-silicon chains have fewer substituents)
IR-6.3.2
Hydrogen substitution in boron hydrides
lR
ec
The construction of names of derivatives of boron hydrides where hydrogen atoms have been
replaced by substituent groups follows the procedures given in Section IR-6.3.1. The only
special feature is the need for specifying replacement of a bridging hydrogen atom, in which
case the designator 'µ-' is used in front of the substituent group name, as in Example 4 below.
na
Examples:
1.
F2B
isi
o
B
BF2
F2B
1
5
2
4
3
IU
PA
C
Pr
2.
ov
2-(difluoroboranyl)-1,1,3,3-tetrafluorotriborane(5)
= CH3
Page 27 of 34
DRAFT 2 April 2004
=F
28
tio
n
s
2-fluoro-1,3-dimethylpentaborane(9), or
2-fluoro-1,3-dimethyl-2,3:2,5:3,4:4,5-tetra-µH-nido-pentaborane(9)
om
me
n
da
3.
= NH2
diboran(6)amine
= NH2
diboran(6)-µ-amine
isi
o
na
lR
ec
4.
NAMES OF IONS AND RADICALS DERIVED FROM PARENT HYDRIDES
ov
IR-6.4
PA
C
Pr
This Section presents names of ions and radicals that can be formally derived from hydrides
by the operations of removal or addition of hydrogen atoms, hydride ions or hydrons. A great
many ions and radicals can also be named by additive methods, as described in Chapter IR-7.
Many simple ions and radicals are named in Table IX, often by both nomenclature types.
IU
IR-6.4.1
Cations derived from parent hydrides by addition of one or more hydrons
The name of an ion formally derived by adding a hydron to a parent hydride is obtained by
adding the suffix 'ium' to the name of the parent hydride, with elision of a final 'e'. For
polycations formed in this way, the suffixes 'diium', 'triium', etc., are used without elision of
any final 'e' on the parent hydride name. Any necessary locants are placed immediately
preceding the suffix. Locants for added hydrons take precedence over locants for
unsaturation, as in Example 8 below.
DRAFT 2 April 2004
Page 28 of 34
29
IR-6.4.2
azanium, or ammonium
diazanium, or hydrazinium
diazanediium, or hydrazinediium
oxidanium, or oxonium
oxidanediium
dioxidanium
triphosphane-1,3-diium
triaz-2-en-1-ium
da
NH4+
N 2H 5+
N2H62+
H 3O +
H4O2+
H 3O 2+
H3PPHPH3+
+H 3N-N=NH
om
me
n
Examples:
1.
2.
3.
4.
5.
6.
7.
8.
tio
n
s
The alternative names ammonium, hydrazinium, hydrazinediium and oxonium are used for
naming organic derivatives, see Section IR-6.4.3 and Section P-73.1.1 of Ref. 1
Cations derived from parent hydrides by loss of one or more hydride ions
na
lR
ec
A cation produced by formal loss of a hydride ion from a parent hydride is named by adding
the suffix 'ylium' to the parent name, with elision of a final 'e'. (See also, Section P-73.2.2.1 of
Ref. 1). For polycations formed in this way, the suffixes 'diylium', 'triylium', etc., are used
without elision of any final 'e' on the parent hydride name. Any necessary locants are placed
immediately preceding the suffix. Locants for removed hydride ions take precedence over
locants for unsaturation, as in Example 5 below.
PH2+
Si2H5+
SiH3+
BH2+
+HN−N=NH
PA
C
Pr
Examples:
1.
2.
3.
4.
5.
ov
isi
o
For the names silane, germane, stannane, plumbane and borane, as well as a number of
hydrocarbon names, 'ylium' replaces the ending 'ane' of the parent hydride. (See also Section
P-73.2.2.1 of Ref. 1).
IU
IR-6.4.3
Page 29 of 34
phosphanylium
disilanylium
silylium
boranylium
triaz-2-en-1-ylium
Substituted cations
Names of substituted derivatives of cations are formed from the modified parent hydride
names (as described in IR-6.4.1 and IR-6.4.2) by adding appropriate substituent prefixes.
DRAFT 2 April 2004
30
IR-6.4.4
tetrafluoroazanium, or tetrafluoroammonium
tetrachlorophosphanium
tetramethylazanium, or tetramethylammonium
[SEtMePh]+
[MeOH2]+
[ClPHPH3]+
ethyl(methyl)phenylsulfanium
methyloxidanium, or methyloxonium
2-chlorodiphosphan-1-ium
da
4.
5.
6.
[NF4]+
[PCl4]+
[NMe4]+
om
me
n
Examples:
1.
2.
3.
tio
n
s
When numbering derivatives of polynuclear parents, the locants for added hydrons or
removed hydride ions take precedence over locants for substituents, as in Example 6 below.
Anions derived from parent hydrides by loss of one or more hydrons
lR
ec
An anion formally obtained by removal of one or more hydrons from a parent hydride is
named by adding 'ide', 'diide', etc., to the parent name, with elision of a terminal 'e' before 'ide'
but not in any other cases. Any necessary locants are placed immediately preceding the
suffix. Locants for removed hydrons take precedence over locants for unsaturation, as in
Example 10 below. (See also Section P-72.2.2 of Ref. 1).
Examples:
2.
H2NNH
_
H2NN2
_
_
HNNH
_
SiH3
_
GeH3
_
SnH3
_
SH
−HN−N=NH
isi
o
4.
5.
Pr
ov
6.
8.
phosphanediide
_
3.
7.
azanide, or amide
na
_
NH2
_
HP2
1.
diazane-1,1-diide
diazane-1,2-diide
silanide
germanide
stannanide
sulfanide
triaz-2-en-1-ide
PA
C
9.
10.
diazanide, or hydrazinide
IU
Names of anions derived by formal loss of one or more hydrons from hydroxy groups and
their chalcogen analogues (characterized by suffixes such as 'ol' and 'thiol') are formed by
adding the ending 'ate' to the appropriate name. (See also, Section P-72.2.2.2 of Ref. 1).
Examples:
11.
12.
_
SiH3O
_
PH2S
silanolate
phosphanethiolate
DRAFT 2 April 2004
Page 30 of 34
31
Anions derived from parent hydrides by addition of one or more hydride ions
da
IR-6.4.5
tio
n
s
The compound in Example 12 may also be named as a derivative of phosphinothious acid,
H2PSH, thus giving the name 'phosphinothioite'. This type of name is used as the basis for
naming organic derivatives of H2PSH. (See discussion of inorganic acids in Chapter IR-8).
om
me
n
The addition of a hydride ion to a parent hydride is designated by the ending 'uide' (see
Section P-73.2 of Ref. 1). Rules regarding locants are analogous to the rules for the 'ide'
suffix (Section IR-6.4.4). For compounds of this kind, additive names (Chapter IR-7) are
common and acceptable alternatives.
Example:
IR-6.4.6
_
[BH4]
boranuide (from borane), or
tetrahydridoborate(1−) (additive)
ec
1.
Substituted anions
1.
_
SnCl3
2.
ov
Examples:
isi
o
na
lR
Names of substituted derivatives of anions are formed from parent hydride names modified
as above (see Sections IR-6.4.4 and IR-6.4.5) by further adding appropriate prefixes for the
substituents. When numbering the structure, the position where a hydron was removed or a
hydride ion was added takes precedence over the positions with substituents, as in Example 4
below. In many cases, additive names are common and acceptable alternatives.
Pr
CH3PH
PA
C
3.
IU
4.
Page 31 of 34
_
CH3NH
trichlorostannanide (from stannane),
or trichloridostannate(1−) (additive)
methylphosphanide
_
methylazanide, or methanaminide
(see Section P-72.2.2.3 of Ref. 1)
9 8 7
6 5
4 3
2 1
Cl3SnOSnH2OSnH2OSnH2OSnH2
_
9,9,9-trichloropentastannoxan-1-ide
5.
[BH3CN]
6.
[PF6]
_
_
cyanoboranuide (from borane),
or cyanidotrihydridoborate(1−) (additive)
hexafluoro-λ5-phosphanuide (from phosphane),
or hexafluoridophosphate(1−) (additive)
DRAFT 2 April 2004
32
Radicals and substituent groups
s
IR-6.4.7
tio
n
Radicals and substituent groups derived from parent hydrides by removal of one or more
hydrogen atoms are named by modifying the parent hydride name as follows:
da
(i) removal of one hydrogen atom: add suffix 'yl' (eliding final 'e' of parent hydride name);
om
me
n
(ii) removal of two or more hydrogen atoms: add suffix 'yl' with appropriate multiplicative
prefix (no vowel elision).
The suffix 'ylidene' is used on a substituent group if a double bond is implied when a skeletal
atom has formally lost two hydrogen atoms. If a triple bond is implied, the ending 'ylidyne' is
used. With these endings, the ending 'e' of the parent hydride name is again elided.
ec
For radicals, if two hydrogens are removed from the same atom the suffix 'ylidene' is used.
na
lR
Locants may be needed to indicate the skeletal atoms from which hydrogen atoms have been
removed. Such locants are placed immediately before the suffix. When numbering the
structure, the positions where hydrogen atoms were removed take precedence over
unsaturation, as in Example 10 below.
isi
o
Radicals may also be named using additive nomenclature, see Section IR-7.1.4.
3.
HP<
P≡
SiH22•
H2Br• and H2Br−
NH2NH• and H2NHN−
•NHNH• and −NHNH−
PA
C
Pr
4.
5.
6.
7
NH2•
PH2• and H2P−
PH2• and HP=
ov
Examples:
1.
2.
8.
9.
IU
10.
azanylidene
phosphanyl
phosphanylidene
phosphanediyl
phosphanylidyne
silanylidene
λ3-bromanyl
diazanyl or hydrazinyl
diazane-1,2-diyl or hydrazine-1,2-diyl
HP=NP•NHPH• and HP=NPNHPH
triphosphaz-4-ene-1,3-diyl
In a number of cases, the established name of a substituent group/radical is non-systematic or
is a shorter version obtained by replacing the ending 'ane' of the parent name by the suffix
'yl':
DRAFT 2 April 2004
Page 32 of 34
tio
n
hydroxyl (for oxidanyl)
hydroxy (for oxidanyl)
aminyl (for azanyl)
amino (for azanyl)
methylidene (for methanylidene)
silyl (for silanyl)
germyl (for germanyl)
stannyl (for stannanyl)
plumbyl (for plumbanyl)
da
OH •
OH−
NH 2•
NH2−
CH22•
SiH3• and SiH3−
GeH3• and GeH3−
SnH3• and SnH3−
PbH3• and PbH3−
om
me
n
Examples:
11.
12.
13.
14.
15.
16.
17.
18.
19.
s
33
This list is exhaustive as far as non-carbon parent hydrides are concerned. A number of
established shortened or entirely non-systematic names are also used for carbon-based
hydrides: methyl, ethyl, propyl, butyl, pentyl, hexyl, cyclohexyl, phenyl, naphthyl, etc.
Substituted radicals or substituent groups
ec
IR-6.4.8
PA
C
3.
IU
IR-6.4.9
Page 33 of 34
aminooxidanyl
hydroxyazanyl
HONH−
hydroxyamine
•
Me3PbPbMe2 and Me3PbPbMe2−
Pr
2.
NH2O• and NH2O−
HONH •
ov
Examples:
1.
isi
o
na
lR
Radicals or substituent groups formally derived by removing one or more hydrogen atoms
and introducing substituents in parent hydrides are named using prefixes for the substituents
as explained in Section IR-6.3.1. The positions from which hydrogen atoms were removed
take priority over the positions with substituents. Several simple such radicals and substituent
groups are named in Table IX. In a few cases the name of a radical and the corresponding
substituent group as used in organic nomenclature may differ (see Example 2 below).
1,1,2,2,2-pentamethyldiplumban-1-yl
(not 1,1,1,2,2-pentamethyldiplumban-2-yl)
Anionic and cationic centres and radicals in a single molecule or substituent group
If several of the above features [cationic moiety, anionic moiety, radical formed by removal of
hydrogen atom(s)] are present in a molecule or a substituent group, a priority rule is needed
to decide in which order to cite the various modifications of the parent hydride name.
DRAFT 2 April 2004
34
The order is:
s
cation < anion < radical < free valence in substituent group
tio
n
in the sense that:
(i) the suffixes indicating these modifications are cited in that order,
3
4.
isi
o
IU
PA
C
5.
New Blue Book
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific
Publications, Oxford, 1990.
Nomenclature of Inorganic Chemistry II, Recommendations 2000, Royal Society of
Chemistry, 2001.
K. Wade, Adv. Inorg. Chem. Radiochem., 18, 1 (1976); R.E. Williams, Adv. Inorg.
Chem. Radiochem., 18, 67 (1976); D.M.P. Mingos, Acc. Chem. Res., 17, 311 (1984)
R.W. Rudolph and W.R. Pretzer, Inorg. Chem., 11, 1974 (1972); R.W. Rudolph, Acc.
Chem. Res., 9, 446 (1976).
ov
3.
1
MeN=N•+−N−−SiMe3
3-methyl-1-(trimethylsilyl)triaz-2-en-2-ium-1-id-2-yl
REFERENCES
1.
2.
2
1,2,2,2-tetramethyldiazan-2-ium-1-ide
Pr
IR-6.5
1
Me3N+−N−−Me
ec
4.
2
tellaniumyl
tellanuidyl
lR
3.
H2Te•+
H2Te•−
na
Examples:
1.
2.
om
me
n
da
(ii) numbering, if necessary, is started at the position or positions of free valences: in
radical species the next lowest locants are given to positions where hydrogen atoms
have been removed; then anion sites are numbered; finally cationic sites are
numbered. All these take precedence over unsaturation and over substituents cited by
prefixes.
DRAFT 2 April 2004
Page 34 of 34
Table IR-6.1 Parent names of mononuclear hydrides
BH3
borane
CH4
methanec
NH3
azaned
H 2O
oxidanea,d
AlH3
alumanea
SiH4
silane
PH3
phosphanee
H 2S
sulfanea,f
GaH3
gallane
GeH4
germane
AsH3
arsanee
InH3
indiganeb
SnH4
stannane
SbH3
stibanee
TlH3
thallane
PbH4
plumbane
BiH3
bismuthanea
d
n
e
m
m
o
c
H2Se
selanea,f
H2Te
H2Po
s
n
o
i
at
HF
HCl
fluoraneg
chloraneg
HBr
bromaneg
tellanea,f
HI
iodaneg
polanea,f
HAt
astataneg
a The names aluminane, bismane, oxane, thiane, selenane, tellurane and polonane cannot be used since they are the names of saturated six-
membered heteromonocyclic rings based on the Hantzsch-Widman system (see Section IR-6.2.4.3). The name 'alane' has been used for
AlH3, but must be discarded because the systematically derived name of the substituent group −AlH2 would be 'alanyl' which is the well
e
R
established name of the acyl group derived from the amino acid alanine.
l
a
b The analogous systematic name for InH3 would be 'indane' which is, however, well established as the name of the hydrocarbon 2,3-
n
io
dihydroindene. The name 'indiane' would lead to confusion when naming unsaturated derivatives, e.g. 'triindiene' could mean a compound with
two double bonds (a diene) as well as the monounsaturated derivative of triindiane. The parent name 'indigane' derives from the etymological
source 'indigo' (from the flame colour of indium).
s
i
v
o
r
P
c The systematic analogue is 'carbane'. Because of the universal use of the name 'methane' for CH4, 'carbane' is not recommended.
d The names 'azane' and 'oxidane' are only intended for use in naming derivatives of ammonia and water, respectively, by substitutive
C
A
P
U
nomenclature. Examples of such use may be found in Section IR-6.4. In Section P.62 of Ref. 1 many organic derivatives of ammonia are
named on the basis of the substituent group suffixes 'amine' and 'imine'.
e The systematic names 'phosphane', 'arsane' and 'stibane' are used throughout this book. The names 'phosphine', 'arsine' and 'stibine' are not
I
recommended.
Page 1 of 2
DRAFT 2 April 2004
f Sulfane, when unsubstituted, may also be named 'hydrogen sulfide' or, better, 'dihydrogen sulfide' (compositional nomenclature, Chapter IR-
s
n
5). However, a compositional name cannot be used as a parent name. Corresponding remarks apply to selane, tellane, and polane.
o
i
at
g The names 'fluorane', 'chlorane', 'bromane', 'iodane' and 'astatane' are included here because they are the basis for the formation of
substitutive names of ions, radicals and substituent groups (see IR-6.4.7 and Table IX for examples). The unsubstituted hydrides may also be
named 'hydrogen fluoride', 'hydrogen bromide', etc. (compositional nomenclature, Chapter IR-5). However, these compositional names cannot
be used as parent names.
d
n
e
m
m
o
c
l
a
e
R
n
io
s
i
v
I
C
A
P
U
o
r
P
DRAFT 2 April 2004
Page 2 of 2
1
IR-7
Additive Nomenclature (Draft March 2004)
tio
n
s
CONTENTS
IR-7.1.1
General
na
INTRODUCTION
isi
o
IR-7.1
lR
ec
om
me
n
da
IR-7.1 Introduction
IR-7.1.1 General
IR-7.1.2 Choosing a central atom or atoms, or a chain or ring structure
IR-7.1.3 Representing ligands in additive names
IR-7.1.4 Ions and radicals
IR-7.2 Mononuclear compounds
IR-7.3 Polynuclear compounds
IR-7.3.1 Symmetrical dinuclear compounds
IR-7.3.2 Non-symmetrical dinuclear compounds
IR-7.3.3 Oligonuclear compounds
IR-7.4 Inorganic chains and rings
IR-7.4.1 General
IR-7.4.2 Nodal descriptor
IR-7.4.3 Name construction
IR-7.5 References
Pr
ov
Additive nomenclature was originally developed for Werner-type coordination compounds,
which were regarded as composed of a central atom (or atoms) surrounded by added groups
known as ligands, but many other types of compound may also be conveniently given additive
names. Such names are constructed by placing the names of the ligands (sometimes
modified) as prefixes to the name(s) of the central atom(s).
IU
PA
C
This Chapter deals with the general characteristics of additive nomenclature and provides
examples of additive names for simple mononuclear and polynuclear compounds. Chain and
ring compounds are then treated using additive principles supplemented by further
conventions. Additive names for inorganic acids are discussed in Chapter IR-8. Additive
nomenclature as applied to metal coordination compounds is described in further detail in
Chapter IR-9. Additive names for a large number of simple compounds are given in Table
IX*.
* Tables numbered with a Roman numeral are collected together at the end of this book.
Page 1 of 15
DRAFT 2 April 2004
2
Choosing a central atom or atoms, or a chain or ring structure
da
IR-7.1.2
tio
n
s
Note that in some cases, compounds named additively may alternatively and equally
systematically be named substitutively (Chapter IR-6). It is important to note, however, that
additive names for parent hydrides cannot be used as parent names in substitutive
nomenclature.
om
me
n
Making a choice of central atom or atoms is a key step in the process of naming a compound
using additive nomenclature. If there is/are (a) metal atom(s) in the compound, it/they should
be chosen as the central atom(s). Such atom(s) should also be relatively central in the
structure and, where possible, should be chosen to make use of molecular symmetry (thereby
shortening the name).
lR
ec
For some compounds, a choice of central atom or atoms will remain. If a choice has to made
between atoms in order to select one as the central atom, the atom that occurs latest when
following the arrow in Table VI should be chosen as the central atom (except that oxygen is
regarded as between chlorine and fluorine in this context).
na
If there is more than one central atom in a structure according to the above criteria then the
compound can be named as a binuclear or polynuclear compound.
Representing ligands in additive names
ov
IR-7.1.3
isi
o
As an alternative to the procedure above, a group of atoms forming a chain or ring substructure within a compound may be chosen in order to give the compound an additive name
using the 'chains and rings' nomenclature outlined in Section IR-7.4.
PA
C
Pr
Additive names are constructed by placing (sometimes modified) ligand names as prefixes to
the name of the central atom.
IU
For anionic ligands, the anion endings 'ide', 'ate' and 'ite' (cf. Section IR-5.3.3) are changed to
'ido', 'ato' and 'ito', respectively, when generating the prefix for the central atom, except in a few
special cases, most notably water (prefix 'aqua'), ammonia (prefix 'ammine'), carbon
monooxide (prefix 'carbonyl'), and nitrogen monooxide (prefix 'nitrosyl').
In principle, it is a matter of convention whether a ligand is considered to be anionic, neutral
or cationic. The default is to consider ligands as anionic, so that OH, for example, receives the
prefix 'hydroxido', Cl the prefix 'chlorido', SO4 the prefix 'sulfato', etc. Some ligands are
DRAFT 2 April 2004
Page 2 of 15
3
s
conventionally regarded as neutral, (e.g. amines and phosphanes, ligands derived from
hydrocarbons by removal of a hydrogen atom, such as methyl, benzyl, etc.).
Ions and radicals
da
IR-7.1.4
tio
n
Appropriate prefixes to represent many simple ligands within names are given in Table IX.
For further details, see Section IR-9.2.2.3.
IR-7.2
om
me
n
Anionic species take the ending 'ate' in additive nomenclature, whereas no distinguishing termination
is used for cationic or neutral species. Additive names of ions end with the charge number (see Section IR5.4.2.2). In additive names of radicals, the radical character of the compound is indicated by a radical dot, •,
added in parentheses and centred, after the name of the compound. Polyradicals are indicated by the
appropriate numerical prefix to the dot. For example, a diradical is indicated by '(2•_'.
MONONUCLEAR COMPOUNDS
isi
o
na
lR
ec
Names of mononuclear compounds, i.e. of species with a single central atom, are formed by
citing the appropriate prefixes for the ligands alphabetically before the name of the central
atom. Ligands occurring more than once are collected in the name by means of multiplicative
prefixes (Table IV), i.e. 'di', 'tri', 'tetra', etc., for simple ligands such as chlorido, benzyl, aqua,
ammine and hydroxido, and 'bis', 'tris', 'tetrakis', etc., for more complex ligands e.g. 2,3,4,5,6pentachlorobenzyl and triphenylphosphane. The latter prefixes are also used to avoid any
ambiguity which might attend the use of 'di', 'tri', etc. Multiplicative prefixes which are not
inherent parts of the ligand name do not affect the alphabetical ordering.
Pr
ov
Prefixes representing ligands can be separated using enclosing marks (see also, Section IR9.2.2.3), and this should be done for all but the simplest ligands, including organic ligands. In
some cases the use of enclosing marks is essential in order to avoid ambiguity, as in
Examples 10 and 11 below.
IU
PA
C
In several of the examples below, substitutive names (see Chapter IR-6) are also given. In
some cases, however, there is no parent hydride available for the construction of a substitutive
name (see Examples 9 and 11). Note also that formulae given below in square bracketes are
coordination compound-type formulae with the central atom listed first.
Examples:
1.
2.
Page 3 of 15
Si(OH)4
tetrahydroxidosilicon (additive),
B(OMe)3
or silanetetrol (substitutive)
trimethoxidoboron or
DRAFT 2 April 2004
4
FClO = [ClFO]
4.
ClOCl = [OCl2]
[Ga{OS(O)Me}3]
or dichlorooxidane (substitutive)
tris(methanesulfinato)gallium (additive),
tio
n
da
5.
s
3.
tris(methanolato)boron (both additive),
or trimethoxyborane (substitutive)
fluoridooxidochlorine (additive),
or fluoro-λ3-chloranone (substitutive)
dichloridooxygen (additive),
6.
7.
om
me
n
or tris(methanesulfinyloxy)gallane (substitutive)
MeP(H)SiH3
trihydrido(methylphosphanido)silicon (additive),
= [SiH3{P(H)Me}] or methyl(silyl)phosphane (substitutive)
NH 2•
hydridonitrogen(2•) (additive),
or azanylidene (substitutive)
hydroxidooxidocarbon(•) (additive),
HOCO•
9.
10.
11.
12.
or hydroxyoxomethyl (substitutive)
FArH = [ArFH]
fluoridohydridoargon (additive)
[HgMePh]
methyl(phenyl)mercury
[Hg(CHCl2)Ph]
(dichloromethyl)phenylmercury (additive)
[Te(C5H9)Me(NCO)2]
ov
isi
o
na
lR
ec
8.
Te
NCO
NCO
bis(cyanato-N)cyclopentyl(methyl)tellurium (additive),
or cyclopentyl[bis(isocyanato)](methyl)-λ4-tellane
(substitutive)
Pr
IU
PA
C
13.
14.
15.
16.
17.
Me
[Al(POCl3)6]3+
[Al(OH2)6]3+
[H(py)2]+
[H(OH2)2]+
[BH2(py)2]+
hexakis(trichloridooxidophosphorus)aluminium(3+)
hexaaquaaluminium(3+)
bis(pyridine)hydrogen(1+)
diaquahydrogen(1+)
dihydridobis(pyridine)boron(1+) (additive),
or bis[pyridine)(N-B)]boranylium (substitutive)
fluoridotrioxidophosphate(2_)
2_
18.
[PFO3]
19.
[Sb(OH)6]
_
hexahydroxidoantimonate(1_) (additive),
or hexahydroxy-λ3-stibanuide (substitutive)
DRAFT 2 April 2004
Page 4 of 15
5
_
difluoridohydrogenate(1_)
[HF2]
21.
[BH2Cl2]
dichloridodihydridoborate(1_) (additive),
OCO•−
NO(2•)−
PO3•2−
or dichloroboranuide (substitutive)
dioxidocarbonate(•1−)
oxidonitrate(2•1−)
trioxidophosphate(•2−)
dichloridoiodine(1+) (additive),
27.
28.
tio
n
or dichloroiodanium (substitutive)
tetrahydridoborate(1_) (additive),
_
or boranuide (substitutive)
pentahydridocarbonate(1_) (additive),
_
or methanuide (substitutive)
hexahydridophosphate(1_) (additive),
CH5
[PF6]
or λ5-phosphanuide (substitutive)
hexafluoridophosphate(1_) (additive),
_
ec
29.
_
BH4
PH6
da
26.
[ICl2]+
om
me
n
22.
23.
24.
25.
_
s
20.
lR
or hexafluoro-λ5-phosphanuide (substitutive)
POLYNUCLEAR COMPOUNDS
IR-7.3.1
Symmetrical dinuclear compounds
na
IR-7.3
ov
isi
o
In symmetrical dinuclear compounds each of the central atoms is of the same type and they
are identically ligated. Two general additive methods of naming are available. Again, in some
cases substitutive names are also easily constructed, as exemplified below.
PA
C
Pr
(i) The ligands are represented in the usual way and the multiplicative affix 'di' is added
immediately before the name of the central atom. The name of the central element is modified
to the 'ate' form if the compound is an anion. A bond between the two central atoms is
indicated by adding to the name the italicized symbols for those two atoms, separated by an
'em' dash and enclosed in parentheses.
IU
Examples:
1.
2.
3.
4.
Page 5 of 15
[Et3PbPbEt3]
hexaethyldilead(Pb—Pb) (additive), or
HSSH •−
1,1,1,2,2,2-hexaethyldiplumbane (substitutive)
dihydridodisulfate(S−S)(•1−) (additive), or
NCCN
NCCN•−
disulfanuidyl (substitutive)
dinitridodicarbon(C—C)
dinitridodicarbonate(C—C)(•1−)
DRAFT 2 April 2004
6
(NC)SS(CN)
6.
(NC)SS(CN)•−
bis(nitridocarbonato)disulfur(S—S), or
dicyanidodisulfur(S—S)
bis(nitridocarbonato)disulfate(S—S)(•1−), or
dicyanidodisulfate(S—S)(•1−)
tio
n
s
5.
Examples:
7.
8.
da
(ii) Alternatively, the name is formed by starting with 'bis' and then citing the name of the halfmolecule in parentheses. Thus, Examples 1-6 become:
bis(triethyllead)(Pb—Pb)
bis(hydridosulfate)(S—S)(•1−) (additive)
9.
10.
NCCN
NCCN•−
bis(nitridocarbon)(C—C)
bis(nitridocarbonate)(C—C)(•1−)
11.
(NC)SS(CN)
12.
(NC)SS(CN)•−
bis{(nitridocarbonato)sulfur}(S—S), or
bis(cyanidosulfur) (S—S)
bis{(nitridocarbonato)sulfate}(S—S) (•1−), or
bis(cyanidosulfate)(S—S)(•1−)
ec
om
me
n
[Et3PbPbEt3]
HSSH •−
na
lR
Note that the last five compounds may also easily be named as chain compounds, as shown in
Section IR-7.4.
isi
o
Note also that the names in Examples 11 and 12 differ from those given in Ref. 1. The
present names are chosen because the sulfur atoms are more obviously the central atoms.
ov
Yet another possibility is to view the compounds as bridged dinuclear species. Bridging
ligands are indicated by the Greek letter µ, placed before the ligand name and separated from
it by a hyphen. The whole term, e.g. 'µ-chlorido', is separated from the rest of the name by
Pr
hyphens. If the bridging ligand occurs more than once, multiplicative prefixes are employed
(see also Sections IR-9.1.2.10 and IR-9.2.5.2).
PA
C
Examples:
13.
ClOCl
µ-oxido-dichlorine
IU
14.
Cl
Cl
Al
Cl
Cl
Al
Cl
Cl
Al2(µ-Cl)2Cl4 or Cl2Al(µ-Cl)2AlCl2
DRAFT 2 April 2004
Page 6 of 15
7
15.
µ-disulfanediido-bis(nitridocarbon)
[NCSSCN]•−
µ-disulfanediido-bis(nitridocarbonate)(•1−)
da
16.
[NCSSCN]
tio
n
s
di-µ-chlorido-tetrachloridodialuminium,
or di-µ-chlorido-bis(dichloridoaluminium)
IR-7.3.2
om
me
n
Note that the names given in Ref. 1 for the species in Examples 6, 12 and 16 were constructed
in a format not used in the present recommendations and thus differ from the names given
here.
Non-symmetrical dinuclear compounds
lR
ec
There are two types of non-symmetrical dinuclear compounds: (i) those with identical central
atoms differently ligated, and (ii) those with different central atoms. In both cases names are
formed by means of the procedure described in Section IR-9.2.5.5, which also deals with
bridging groups.
isi
o
na
Priority is assigned to the central atoms as follows. For cases of type (i) the central atom
carrying the greater number of alphabetically preferred ligands is numbered 1. For cases of
type (ii) the number 1 is assigned to the higher priority central element of Table VI, whatever
the ligand distribution.
ov
In both types of compound, names are constructed in the usual way, by first citing the
prefixes representing the ligands, in alphabetical order. Each prefix representing a ligand is
followed by a hyphen, the number assigned to the central atom to which the ligand is attached,
the Greek letter κ (kappa) (see Section IR-9.2.4.2) with a right superscript denoting the
IU
PA
C
Pr
number of such ligands bound to the central atom (the number 1 being omitted for a single
ligand), and the italic capital element symbol for the ligating atom by which the ligand is
attached to the central atom. This describes the ligands and their mode of attachment. The
κ construction can be omitted in very simple cases (see Examples 1 and 2 below).
The central atoms are listed after the ligands. The multiplicative prefix 'di' is used where the
central atoms are the same element. Otherwise, the order of the central atoms is obtained
using Table VI (except that in this context oxygen is regarded by custom as being placed
between chlorine and fluorine). The order of the central atoms reflects the numbering
employed with the κ symbols. The ending 'ate' is added if the dinuclear compound is an
anion, and a radical dot may be added for radicals. In the case of two different central atoms,
Page 7 of 15
DRAFT 2 April 2004
8
2.
ClOO•
3.
ClClF+
oxido-1κO-dichlorine(Cl—Cl),
tio
n
ClClO
or simply oxidodichlorine
chlorido-1κCl-dioxygen(O—O)(•),
or simply chloridodioxygen(•)
fluorido-1κF-dichlorine(Cl—Cl)(1+),
da
Examples:
1.
s
the two names are cited in alphabetical order inside parentheses and 'ate' is added outside the
parentheses.
4.
[O3POSO3]
om
me
n
or simply fluoridodichlorine(1+)
2_
µ-oxido-hexaoxido-1κ3O,2κ3O-(phosphorussulfur)ate(2−),
or simply µ-oxido-hexaoxido(phosphorussulfur)ate(2−)
1
ec
5.
Me3Sn
BiMeEt
lR
S
2
ethyl-2κC-tetramethyl-1κ3C,2κC-µ-thiophene-2,5-diyl-tinbismuth
na
6.
2 1
ov
7.
isi
o
[Cl(HPhN)2GeGeCl3]
tetrachlorido-1κ3Cl,2κCl-bis(phenylamido-2κN)-digermanium(Ge—Ge)
1 2
triphenyl-2κ3C-lithiumlead(Li—Pb)
Pr
LiPbPh3
PA
C
Where the precise positions of ligation are unknown, or are known to be mixed, the situation
is met appropriately by use of names in the style of Section IR-7.3.1.
IU
Examples:
8.
9.
IR-7.3.3
[Pb2(CH2Ph)2F4]
dibenzyltetrafluoridodilead
[Ge2(CH2Ph)Cl3(NHPh)2]
(benzyl)trichloridobis(phenylamido)digermanium
Oligonuclear compounds
DRAFT 2 April 2004
Page 8 of 15
9
HO3•
hydrido-1κH-trioxygen(•), or simply hydridotrioxygen(•)
HON 3•−
hydroxido-1κO-trinitrate(2N—N)(•1−)
Cl3SiSiCl2SiCl3
da
Examples:
1.
2.
3.
tio
n
s
In simple cases, the principles of the preceding sections may be generalised for the naming of
oligonuclear compounds. Again, there are compounds which are also easily named by
substitutive nomenclature because of the availability of obvious parent hydrides.
4.
om
me
n
octachloridotrisilicon(2 Si—Si) (additive), or
octachlorotrisilane (substitutive)
FMe2SiSiMe2SiMe3
1κF-fluoridoheptamethyltrisilicon(2 Si—Si) (additive), or
1-fluoro-1,1,2,2,3,3,3-heptamethyltrisilane (substitutive)
ec
(An alternative additive name for the compound in Example 3, based on the longest chain in
the molecule, can also be constructed by the method described in Section IR-7.4.3).
isi
o
Me3SiSeSiMe3
µ-selenidobis(trimethylsilicon) (additive), or
hexamethyl-1κ3C,3κ3C-2-selenium-1,3-disilicon(2 Si—Se) (additive),
ov
Example:
5.
na
lR
For heterooligonuclear systems, more conventions are needed to identify and name the
collection of central atoms, and to number the central atoms so as to provide locants for the
ligands.
or 1,1,1,3,3,3-hexamethyldisilaselenane (substitutive)
IR-7.4
PA
C
Pr
Note that in the last example one can choose to name the compound as dinuclear or
trinuclear. The complexities deriving from the structural variations which may occur due to
homonuclear and heteronuclear central atom clusters and bridging groups are dealt with in
more detail in Sections IR-9.2.5.6 to IR-9.2.5.8.
IU
IR-7.4.1
Page 9 of 15
INORGANIC CHAINS AND RINGS
General
Inorganic chain and ring compounds can be named2 according to a particular system of
additive nomenclature which does not require prior knowledge about the nature of bonds
between atoms. The method can be applied to all chain and ring compounds but its use is
DRAFT 2 April 2004
10
tio
n
s
principally intended for species mainly composed of atoms other than carbon. While small
compounds can more conveniently be named by using several alternative methods, the
advantage of this nomenclature system lies in the simple way in which complicated structures
can be derived from the name and vice versa.
Examples:
1.
ec
om
me
n
da
A neutral chain compound is called 'catena' preceded by a multiplicative prefix, 'di', 'tri', etc., to
indicate the number of branches in the molecule. Likewise, cyclic compounds are called 'cycle'
preceded by the appropriate multiplicative prefix. A mixed chain and ring compound is named
as 'catenacycle'. In the case of cationic and anionic species the names are modified by the
suffixes 'ium' and 'ate', respectively. Radical species may be further specified using the radical
dot (Section IR-7.1.4).
na
lR
2.
dicycle
Pr
PA
C
IU
ov
isi
o
3.
IR-7.4.2
tricatena
tricatenadicycle
Nodal descriptor
DRAFT 2 April 2004
Page 10 of 15
11
da
tio
n
s
The connectivity in the molecular framework is indicated by a nodal descriptor, which is
placed in square brackets immediately before the terms 'catena', 'cycle' or 'catenacycle'. The
atoms are numbered according to the general nodal nomenclature regardless of their chemical
identity. Only in the case of ambiguity is the chemical nature of the atoms taken into
consideration.
om
me
n
The first part of the descriptor indicates the number of atoms in the main chain. The arabic
numerals after the full stop indicate the lengths of the branches cited in priority order. A
superscript locant for each branch denotes the atom in the part of the molecule already
numbered to which the branch is attached. A zero in the descriptor indicates a ring and is
followed by an arabic numeral indicating the number of atoms in the main ring. The
numbering begins from one of the bridgeheads and proceeds in the direction to give the
lowest possible locant for the other bridgehead. The construction of the descriptor for
assemblies consisting of several modules is treated in detail in Ref. 2.
ec
Examples:
1.
lR
1
7
na
descriptor: [7]
Pr
ov
isi
o
2.
1
PA
C
IU
6
descriptor: [5.13]
3.
Page 11 of 15
3
descriptor: [06]
4.
DRAFT 2 April 2004
5
12
8 s
tio
n
7
1
4
om
me
n
5.
da
descriptor: [07.11,4]
descriptor: [09.01,5]
lR
ec
6.
isi
o
na
Name construction
Pr
IR-7.4.3
ov
descriptor: [(09.01,5)2:20(8.2315)]
PA
C
The atoms forming the nodal skeleton are listed in alphabetical order complete with their
locants and are named using 'y' terms, examples of which are given in Table IR-7.1; a full list
is given in Table X.
IU
Table IR-7.1. Some 'y' terms for naming elements in the nodal framework
H
hydrony
C
carby
N
azy
O
oxy
B
bory
Si
sily
P
phosphy
S
sulfy
DRAFT 2 April 2004
Page 12 of 15
13
germy
As
arsy
Se
seleny
Sn
stanny
Sb
stiby
Te
tellury
tio
n
s
Ge
da
Atoms and groups of atoms which are not a part of the nodal framework are named as ligands
and are cited in alphabetical order, together with their locants, before the cited sequence of the
atoms constituting the nodal framework.
ec
Cl3SiSiCl2SiCl3
2,2,3,3,4,4-hexachlorido-1,5-dichlory-2,3,4-trisily-[5]catena
1,4-dihydrony-2,3-disulfy[4]catenate(•1−)
lR
Examples:
1.
om
me
n
The first six examples below were also named in Section IR-7.3.1. These simple molecules
would be better named by other nomenclature systems, but they illustrate the name
construction. The power of additive ring and chain nomenclature lies in the straightforward
way in which structures of complicated branched-chain and polycyclic molecules can be
arrived at from the name and vice versa. It should be evident that a systematic name of a
complicated molecule will be necessarily long.
2.
HSSH •−
3.
4.
NCCN
NCCN•−
5.
6.
NCSSCN
1,6-diazy-2,5-dicarby-3,4-disulfy[6]catena
•−
NCSSCN
1,6-diazy-2,5-dicarby-3,4-disulfy[6]catenate(•1−)
PA
C
na
isi
o
Pr
ov
7.
1,4-diazy-2,3-dicarby[4]catena
1,4-diazy-2,3-dicarby[4]catenate(•1−)
S
S
12
S
1
13
S
S
S
N
S
S4
S
N
7
S
S
1,7-diazyundecasulfy-[012.11,7]dicycle
IU
Since this compound contains only nitrogen and sulfur, it is not necessary to indicate the
locants of all sulfur atoms. Only the locants of the two nitrogen atoms are needed.
Page 13 of 15
Examples:
8.
DRAFT 2 April 2004
14
5
S
6
7
1
S
P
I
I
P
P
2
3
s
P
tio
n
4
da
S
3,6-diiodido-1,3,4,6-tetraphosphy-2,5,7-trisulfy-[06.11,4]dicycle
om
me
n
9.
Me
N
N
F
7
O
Me
C 6
O
2
P
S 3
1
5
N
ec
N
O
4
Me
lR
Me
na
1-fluorido-2,4,5,7-tetramethyl-3,3,6-trioxido-2,4,5,7-tetraazy-6-carby-1-phosphy-3-sulfy[04.31,1]dicycle
ov
isi
o
Compounds containing both cyclic and acyclic parts are named as assemblies, which are
composed of modules. The descriptors of individual modules are connected by a colon. The
locants on either side of the two colons indicate the points of attachment between the
modules. (For naming assemblies, see Ref. 2).
IU
PA
C
Pr
Examples:
10.
5
S
S
S
S
S8
1
S
N
18
9
10
S
S
S
S
S
N 11
S
S
S 15
S
S
1,11-diazyhexadecasulfy-[(08)1:9(2)10:11(08)]catenadicycle
11.
DRAFT 2 April 2004
Page 14 of 15
15
H
7
B
H
B
B
4
2
H
10
11
N
H H2
H
3
5
CH2
da
H
B
CH2
s
H 6
B
H 2N
tio
n
1
9
8
H
12.
om
me
n
1,2,2,4,6,7,8,8,9,9,10,10,11,11-tetradecahydrido-8,11-diazy-1,2,4,6,7-pentabory-9,10-dicarby3,5-dihydrony-[(06.01,402,404,6)1:7(05)]pentacycle
[LiAl4]-
-
1
Li
5
2
Al
lR
4
Al
ec
Al
Al
3
REFERENCES
Names for Inorganic Radicals, Pure Appl. Chem., 72, 437 (2000).
Nomenclature of Inorganic Chain and Ring Compounds, Pure Appl. Chem., 69,
1659 (1997); Chapter II-5 in Nomenclature of Inorganic Chemistry II,
Recommendations 2000, Royal Society of Chemistry, 2001.
IU
PA
C
Pr
ov
1.
2.
isi
o
IR-7.5
na
tetraaluminy-1-lithy-[05.01,301,402,5]tetracyclate(1-)
Page 15 of 15
DRAFT 2 April 2004
1
IR-8
Inorganic Acids and Derivatives (March 2004)
IR-8.1
tio
n
da
Introduction and overview
General principles for systematic naming of acids
Additive names
Hydrogen names
Abbreviated hydrogen names for certain anions
Functional replacement names for derivatives of oxoacids
References
INTRODUCTION AND OVERVIEW
om
me
n
IR-8.1
IR-8.2
IR-8.3
IR-8.4
IR-8.5
IR-8.6
IR-8.7
s
CONTENTS
na
lR
ec
A number of inorganic and simple carbon-containing compounds are commonly given nonsystematic or semi-systematic names containing the word 'acid'. Examples are boric acid or
orthoboric acid, metaboric acid, fulminic acid, phosphoric acid, diphosphoric acid, cyclotriphosphoric acid, catena-triphosphoric acid, dithionous acid, peroxodisulfuric acid or
peroxydisulfuric acid, etc. These names are unique in modern nomenclature in that,
interpreted literally, they describe a particular chemical property of the compounds in
question. Systematic names are otherwise based solely on composition and structure.
Pr
ov
isi
o
All such acids may also be given structure-based systematic names using principles already
described in preceding chapters on substitutive and additive nomenclature, so in that respect
the 'acid'-containing names are superfluous. Furthermore, many species which would be
classified as acids based on their chemical properties, are never named as such, e.g. aqua ions
such as hexaaquaaluminium(3+), and hydrides and derivatives such as ammonium, hydrogen
sufide (sulfane), etc. The term 'acid' is thus not used consistently.
IU
PA
C
Based on these considerations, the use of the word 'acid' in any new name in inorganic
nomenclature is discouraged. However, a number of the existing 'acid' names are so
commonly used (sulfuric acid, perchloric acid, etc.) that it would be unrealistic to suggest
replacing them altogether by systematic alternatives. Another reason to include them in the
present recommendations is that the acids in question are used as parent structures in the
nomenclature of certain organic (i.e. carbon-containing) derivatives so that the derivative
names are directly or indirectly based on the names containing the word 'acid'. See examples
below and Section IR-8.6.
The main purposes of this chapter are:
Page 1 of 12
DRAFT 2 April 2004
2
tio
n
s
(a) to show how the inorganic species commonly named as acids may be given
systematic additive names (Section IR-8.3 and Tables IR-8.1 and IR-8.2);
(b) to list the 'acid' names that are still allowed due to common usage and/or because
they are needed in organic nomenclature (see also, Tables IR-8.1 and IR-8.2).
om
me
n
da
In addition, Sections IR-8.4 and IR-8.5 deal with a further type of names, denoted here as
hydrogen names. These names can be viewed as generalisations of common anion names
such as 'hydrogencarbonate', but they are not necessary for naming completely specified
molecular structures and can be regarded as a special topic.
ec
It is important to understand that although this chapter provides several acceptable names for
many inorganic acids, it creates some order in those names; it is left to practitioners to choose
the name most suitable for a particular application. In the future, IUPAC aims to provide
preferred names for inorganic species, including the acids dealt with here, just as Ref. 1 does
for organic species.
isi
o
na
lR
Finally, names which do not denote compounds of a definite composition, such as
'hydrochloric acid', 'stannic acid', 'tungstic acid', etc., are outside the scope of the systematic
nomenclature presented here. However, the chemical systems involved can always be
discussed using systematic names such as 'hydrogen chloride', 'tin(IV) oxide', 'tungsten(VI)
oxide', etc.
Pr
ov
A few examples are given now in order to illustrate some of the above general remarks. In
these examples, and in the remainder of this chapter, alternative formulae are sometimes
provided for clarity in connection with the discussion of additive names. These are based on a
perception of the structures in question as generalised coordination entities. For mononuclear
entities, this means that the central atom symbol is listed first and then the ligand symbols in
alphabetical order, as prescribed in Section IR-4.4.3.2.
IU
PA
C
Example:
1.
phosphoric acid = H3PO4 = [PO(OH)3].
Based on the structure, the compound can be named additively (Chapter IR-7) as
trihydroxidooxidophosphorus or substitutively (Chapter IR-6) as a derivative of the parent
hydride λ5-phosphane (PH5), leading to the name trihydroxy-λ5-phosphanone.
As opposed to the two latter names, the name 'phosphoric acid' does not convey the structure,
but does fit into a general pattern whereby the ending 'ic' denotes a higher or the highest
DRAFT 2 April 2004
Page 2 of 12
3
tio
n
trimethyl phosphate
hexamethylphosphoric triamide
PO(OMe)3
PO(NMe2)3
da
Examples:
2.
3.
s
possible oxidation state (compare 'nitric acid', 'sulfuric acid'). Examples 2 and 3 show organic
derivatives named on the basis of phosphoric acid as the parent.
om
me
n
Each of these two compounds could also be named substitutively, on the basis of the above
parent hydride, or additively, but the names given here are preferred IUPAC names (see
Sections P-67.1.2.4 and P-67.1.3.1 of Ref. 1).
Some organic derivative names still contain the word 'acid', as in the following derivatives of
arsonic acid = H2AsHO3 = [AsHO(OH)2].
PhAsO(OH)2
phenylarsonic acid
5.
EtAsCl(OH)S
ethylarsonochloridothioic O-acid
ec
Examples:
4.
na
lR
The name in Example 4 regards the compound as derived from arsonic acid, by substitution
of a phenyl group for the hydrogen atom bound directly to arsenic. The name in Example 5,
in addition to the hydrogen substitution, involves functional replacement nomenclature
(Section IR-8.6).
ov
isi
o
Note that there is one general case where the word 'acid' may appear in a fully systematic
name of an inorganic compound, namely when substitutive nomenclature is used and
prescribes a suffix for the highest ranking substituent group which ends with the word 'acid'.
Consider the polythionic acids, H2SnO6 = [(HO)(O)2SSn−2S(O)2(OH)] (n ≥ 2), which
Pr
have the common names 'dithionic acid', 'trithionic acid', 'tetrathionic acid', etc. They may be
named systematically using additive nomenclature, as shown in Table IR-8.1. For n ≥ 3, they
IU
PA
C
may also be named substitutively on the basis of the central (poly)sulfane skeleton, as
exemplified below.
Page 3 of 12
Examples:
6.
7.
H2S3O6 = [(HO)(O)2SSS(O)2(OH)]
H2S4O6 = [(HO)(O)2SSSS(O)2(OH)]
sulfanedisulfonic acid
disulfanedisulfonic acid,
Table IR-8.1 Acceptable common names and fully systematic (additive) names for
oxoacid and related structures.
DRAFT 2 April 2004
4
GENERAL PRINCIPLES FOR SYSTEMATIC NAMING OF ACIDS
tio
n
IR-8.2
s
(Insert separate landscape Table)
da
Molecular compounds and ions commonly viewed as inorganic acids are treated no
differently from other molecular species when constructing systematic names.
om
me
n
The most easily applied general principle for systematic naming is that of additive
nomenclature, exemplified in Section IR-8.3. As mentioned in IR-8.1, substitutive
nomenclature could also be generally applied. However, this is not further elaborated here.
Sections IR-8.4 and IR-8.5 describe hydrogen names, which are related to additive names and
only needed in special cases.
ADDITIVE NAMES
lR
IR-8.3
ec
The method called 'acid nomenclature' in Section I-9.6 of Ref. 2 is little used and not needed.
Its use is therefore no longer recommended.
isi
o
H3SO4+ = [SO(OH)3]+
H2SO4 = [SO2(OH)2]
HSO4− = [SO3(OH)]−
trihydroxidooxidosulfur(1+)
dihydroxidodioxidosulfur
hydroxidotrioxidosulfate(1−)
ov
Examples:
1.
2.
3.
na
Molecules or ions that can formally be regarded as mononuclear coordination entities may be
named additively, applying the rules described in Chapter IR-7.
IU
PA
C
Pr
Structures which can be regarded as oligonuclear coordination entities may be named as such
(Section IR-7.3) or may be named using the system for inorganic chains and rings (Section
IR-7.4).
In principle, the choice of method in the latter case is arbitrary. However, the machinery of
coordination compound nomenclature was developed to enable the handling of complex
structures involving polyatomic, and particularly polydentate, ligands and sometimes multiply
bridging ligands. Furthermore, the separation into ligands and central atoms, obvious in
compounds most usually classified as coordination compounds, may be less obvious in the
polyoxoacids. Thus, additive nomenclature of the coordination type tends to be more intricate
than necessary when naming polyoxoacids forming relatively simple chains and rings. Here
DRAFT 2 April 2004
Page 4 of 12
5
s
the chains and rings system is easily applied, and the names so derived are easy to decipher.
However, this system can lead to long names with many locants.
The compound commonly named diphosphoric acid, H4P2O7 =
[(HO)2P(O)OP(O)(OH)2], is named according to the coordination-type
additive nomenclature as:
om
me
n
µ-oxido-bis[dihydroxidooxidophosphorus]
da
Examples:
4.
tio
n
Both types of additive names are exemplified below for oligonuclear systems.
or as a five-membered chain with ligands:
1,5-dihydrido-2,4-dihydroxido-2,4-dioxido-1,3,5-trioxy-2,4-diphosphy-[5]catena
The compound commonly named cyclo-triphosphoric acid:
O
P
OH
O
P
lR
HO
ec
5.
O
na
O
O
isi
o
O
P
OH
H 3P 3O 9
ov
may be named according to coordination-type additive nomenclature as:
tri-µ-oxido-tris(hydroxidooxidophosphorus),
Pr
or as a six-membered ring with ligands:
2,4,6-trihydroxido-2,4,6-trioxido-1,3,5-trioxy-2,4,6-triphosphy-[6]cycle
IU
PA
C
6.
The related compound, catena-triphosphoric acid,
O
HO
P
OH
O
O
P
O
O
OH
H5P3O10
Page 5 of 12
DRAFT 2 April 2004
P
OH
OH
6
may be named as a trinuclear coordination entity:
s
pentahydroxido-1κ2O,2κO,3κ2O-di-µ-oxido-trioxido-1κO,2κO,3κO-triphosphorus,
tio
n
or as a symmetrical dinuclear coordination entity with a bridging phosphate
ligand:
µ-[hydroxidotrioxidophosphato(2−)-1κO,2κO]-bis(dihydroxidooxidophosphorus),
da
or as a mononuclear coordination entity with two phosphate ligands:
bis(dihydroxidodioxidophosphato)hydroxidooxidophosphorus,
om
me
n
or as a seven-membered chain with ligands:
1,7-dihydrido-2,4,6-trihydroxido-2,4,6-trioxido-1,3,5,7-tetraoxy-2,4,6-triphosphy-[7]catena.
All inorganic oxoacids for which a common name containing the word 'acid' is still acceptable
according to the present recommendations (and in many cases used as a parent in functional
ec
nomenclature, as described in Section IR-8.1) are listed in Table IR-8.1 together with additive
names to illustrate how systematic names may be given.
na
lR
Several names omitted from Ref. 2, e.g. selenic acid and hypobromous acid, are reinstated
because they are unambiguous and remain in common use (including their use as parent
names in replacement nomenclature).
ov
isi
o
Table IR-8.1 also includes anions derived from the neutral oxoacids by successive
dehydronation. Many of these anions also have common names that are still allowed, in some
cases in spite of the fact that they are based on nomenclature principles that are now
otherwise abandoned (e.g. nitrate/nitrite and perchlorate/chlorate/chlorite/hypochlorite). For
names involving the prefix 'hydrogen', see Sections IR-8.4 and IR-8.5.
IU
IR-8.4
PA
C
Pr
It is important to note that the presence of a structure in Table IR-8.1 does not imply that the
structure has been described in the literature or that there has been a need to name it in the
past. Several names are included only for completeness and to make parent names available
for naming organic derivatives.
HYDROGEN NAMES
An alternative nomenclature for hydrogen-containing compounds and ions is described here.
The word 'hydrogen', with a numerical prefix if relevant, is joined (with no space) to an anion
name formed by additive nomenclature and placed within appropriate enclosing marks (see
Section IR-2.2). This construction is followed (again with no space) by a charge number
DRAFT 2 April 2004
Page 6 of 12
7
s
indicating the total charge of the species or structural unit being named (except for neutral
species/units).
da
tio
n
Hydrogen names are useful when the connectivity (the positions of attachment of the
hydrons) in a hydron-containing compound or ion is unknown or not specified (i.e. when
which of two or more tautomers is not specified, or when one does not wish to specify a
complex connectivity, such as in network compounds).
Examples:
1.
om
me
n
Some of the following examples are discussed in detail below.
H 2P 2O 72−
dihydrogen(heptaoxidodiphosphate)(2−)
2.
H2B2(O2)2(OH)4
3.
H2Mo6O19 = H2[Mo6O19]
4.
H4[SiW12O40] = H4[W12O36(SiO4)]
dihydrogen(tetrahydroxidodi-µ-peroxido-diborate)
dihydrogen(nonadecaoxidohexamolybdate)
5.
lR
ec
tetrahydrogen[(tetracontaoxidosilicondodecatungsten)ate], or
tetrahydrogen[hexatriacontaoxido(tetraoxidosilicato)dodecatungstate], or
tetrahydrogen(silicododecatungstate)
H4[PMo12O40] = H4[Mo12O36(PO4)]
6.
isi
o
na
tetrahydrogen[tetracontaoxido(phosphorusdodecamolybdenum)ate], or
tetrahydrogen[hexatriacontaoxido(tetraoxidophosphato)dodecamolybdate], or
tetrahydrogen(phosphododecamolybdate)
H6[P2W18O62] = H6[W18O54(PO4)2]
H4Fe(CN)6
Pr
7.
ov
hexahydrogen[dohexacontaoxido(diphosphorusoctadecatungsten)ate], or
hexahydrogen[tetrapentacontaoxidobis(tetraoxidophosphato)octadecatungstate], or
hexahydrogen(diphosphooctadecatungstate)
H2PtCl6·2H2O
9.
HCN
PA
C
8.
tetrahydrogen(hexacyanidoferrate)
dihydrogen(hexachloridoplatinate)—water (1/2)
hydrogen(nitridocarbonate)
IU
In Example 1, the two hydrons could be located either on two oxygen atoms on the same
phosphorus atom or one on each of the phosphorus atoms. Thus, as already indicated,
hydrogen names do not necessarily fully specify the structure.
Page 7 of 12
In the same way, the hydrogen name in Example 9 covers, in principle, two tautomers. This
also applies to the common compositional name 'hydrogen cyanide'. The names
'hydridonitridocarbon' (additive nomenclature), 'methylidyneazane' (substitutive
DRAFT 2 April 2004
8
s
nomenclature) and 'formonitrile' (functional organic nomenclature) all specify the tautomer
HCN.
hydrogen(tetraoxidomanganate)
dihydrogen(tetraoxidomanganate)
dihydrogen(tetraaoxidochromate)
hydrogen(tetraoxidochromate)(1−)
dihydrogen(heptaoxidodichromate)
dihydrogen(peroxide)
hydrogen(peroxide)(1−)
dihydrogen(sulfide)
dihydrogen(trioxidonitrate)(1+)
om
me
n
HMnO4
H2MnO4
H2CrO4
HCrO4−
H2Cr2O7
H 2O 2
HO2−
H 2S
H 2NO 3+
ec
Examples:
10.
11.
12.
13.
14.
15.
16.
17.
18.
da
tio
n
Hydrogen names may also be used for molecular compounds and ions with no tautomerism
problems if one wishes to emphasise the conception of the structure as hydrons attached to
the anion in question:
lR
Note the difference from compositional names such as 'hydrogen peroxide' for H2O2 and
'hydrogen sulfide' for H2S (Chapter IR-5) in which (in English) there is a space between the
na
electropositive and electronegative component(s) of the name.
Pr
ov
isi
o
Compositional names of the above type, containing the word 'hydrogen', were classified as
'hydrogen nomenclature' in the discussion of oxoacids in Section I-9.5 of Ref. 2, and such
names were extensively exemplified. However, in order to avoid ambiguity, their general use
is not encouraged here. Consider, for example, that the compositional names 'hydrogen
sulfide' and 'hydrogen sulfide(2−)' can both be interpreted as H2S as well as HS−. The
situation with H2S is completely analogous to that with Na2S which may be named sodium
sulfide, disodium sulfide, sodium sulfide(2−) and disodium sulfide(2−), except that in the
latter case misinterpretation of the first and third names as denoting NaS− is improbable. In
Ref. 2, the names 'hydrogensulfide(1−)' and 'monohydrogensulfide' for HS− were proposed
IU
PA
C
to avoid ambiguity. (However, in some languages there is no space in compositional names
so that very delicate distinctions are required anyway.)
The strict definition of hydrogen names proposed here is meant to eliminate such confusion
by imposing the requirements:
(i) that 'hydrogen' be attached to the rest of the name,
(ii) that the number of hydrogens must be specified by a multiplicative prefix,
(iii) that the anionic part be placed in enclosing marks, and
DRAFT 2 April 2004
Page 8 of 12
9
(iv) that the charge of the total structure being named is specified.
tio
n
s
Hydrogen names constructed in this way cannot be mistaken for other types of name.
da
The only allowed exceptions to the above format for hydrogen names are the few particular
abbreviated anion names listed in Section IR-8.5.
om
me
n
In a few cases, no confusion can arise, and the distinction between compositional name and
hydrogen name is not as important, most notably for the hydrogen halides. Thus, HCl can
equally unambiguously be named 'hydrogen chloride' (compositional name) and
'hydrogen(chloride)' (hydrogen name).
lR
ec
Examples 1, 3-6 and 14 above demonstrate that homo- and heteropolyoxoacids and their
partially dehydronated forms can be given hydrogen names once the corresponding anions
have been named. Examples 4-6 each feature three alternatives. The first two names are both
fully additive for the anion part and correspond to different ways of dissecting the structure
into ligands and central atoms. The last names, involving the prefixes 'silico' and 'phospho',
are examples of a common semi-systematic nomenclature which is not recommended for
general use because it requires complex conventions in order to be unambiguous.
na
Rules for naming very complicated homo- and heteropolyoxoanions are given in Chapter II-1
of Ref. 3.
Pr
ov
isi
o
Note that Examples 10-14 above show how one may easily name transition metal compounds
that have been named as acids in the past. Names such as permanganic acid, dichromic acid,
etc., are not included in the present recommendations because they represent an area where it
is difficult to systematise and decide what to include, and where the names are not needed for
organic nomenclature, as opposed to the corresponding 'acid' names for acids of main group
elements.
IU
IR-8.5
PA
C
Finally, note that usage is different from the above in the names of salts and partial esters of
organic polyvalent acids, where 'hydrogen' is always cited as a separate word just before the
anion name, e.g. 'potassium hydrogen phthalate' or 'ethyl hydrogen phthalate'.
Page 9 of 12
ABBREVIATED HYDROGEN NAMES FOR CERTAIN ANIONS
A few common anionic species have names which can be regarded as short forms of
hydrogen names formed according to the above method. These names, all in one word
without explicit indication of the molecular charge, and without the enclosing marks, are
DRAFT 2 April 2004
10
tio
n
s
accepted due to their brevity and long usage and because they are not ambiguous. It is
strongly recommended that this list be viewed as limiting due to the ambiguities that may
arise in many other cases, cf. the discussion in Section IR-8.4.
Accepted simplified
hydrogen name
Hydrogen name
H2BO3−
HBO32−
HSO4−
HCO3−
H2PO4−
HPO42−
HPHO3−
H2PO3−
HPO32−
HSO4−
HSO3−
dihydrogenborate
hydrogenborate
hydrogensulfate
hydrogencarbonate
dihydrogenphosphate
hydrogenphosphate
hydrogenphosphonate
dihydrogenphosphite
hydrogenphosphite
hydrogensulfate
hydrogensulfite
dihydrogen(trioxidoborate)(1−)
hydrogen(trioxidoborate)(1−)
hydrogen(tetraoxidosulfate)(1−)
hydrogen(trioxidocarbonate)(1−)
dihydrogen(tetraoxidophosphate)(1−)
hydrogen(tetraoxidophosphate)(2−)
hydrogen(hydridotrioxidophosphate)(2−)
dihydrogen(trioxidophosphate)(2−)
hydrogen(trioxidophosphate)(1−)
hydrogen(tetraoxidosulfate)(1−)
hydrogen(trioxidosulfate)(1−)
lR
ec
om
me
n
da
Anion
IR-8.6
FUNCTIONAL REPLACEMENT NAMES FOR DERIVATIVES OF OXOACIDS
na
In functional replacement nomenclature, substitution of =O or −OH groups in parent
oxoacids (such as O → S, O → OO, OH → Cl, etc.) is indicated by the use of infixes as
isi
o
exemplified below (cf. Ref. 1, Section P-67.1.2.1).
Infix
OH → NH2
O → OO
O→S
O → Se
O → Te
OH → F
OH → Cl
OH → Br
OH → I
OH → CN
amid(o)
peroxo
thio
seleno
telluro
fluorid(o)
chlorid(o)
bromid(o)
iodid(o)
cyanid(o)
IU
PA
C
Pr
ov
Replacement operation
DRAFT 2 April 2004
Page 10 of 12
11
Example 5 in Section IR-8.1 demonstrates the use of the prefixes for OH → Cl and O → S
EtAsCl(OH)S
ethylarsonochloridothioic O-acid.
tio
n
s
to arrive at the name 'arsonochloridothioic O-acid' for the derived parent HAsCl(OH)S =
[AsClH(OH)S], required for naming the organic derivative:
Example:
1.
om
me
n
da
Functional replacement names may, of course, be used for the derived parent acids
themselves. However, since this amounts to introducing an additional system which is not
needed, such names are not recommended for general use in inorganic nomenclature. As
mentioned above, additive and substitutive nomenclature can always be used.
HAsCl(OH)S = [AsClH(OH)S]
chloridohydrido(hydroxido)(sulfido)arsenic (additive), or
chloro(hydroxy)-λ5-arsanethione (substitutive)
na
lR
ec
Nevertheless, in Table IR-8.2 several inorganic structures are listed which can be regarded as
derived from structures in Table IR-8.1 by various replacement operations. Acceptable
common names are given which are sometimes based on a variation of the above system
using a prefix instead of an infix (e.g. 'thiosulfuric acid' rather than 'sulfurothioic acid'). The
prefix variant is accepted only in these specific cases.
isi
o
Table IR-8.2 Common names, functional replacement names, and fully systematic (additive)
names for some functional replacement derivatives of oxoacids.
ov
(Insert separate landscape Table)
PA
C
Pr
A problem that would arise with general use of the prefix variant of functional replacement
names is illustrated by the thio acids. The names 'trithiocarbonic acid', 'tetrathiophosphoric
acid', etc., would lead to anion names 'trithiocarbonate', 'tetrathiophosphate', etc., which appear
to be additive names but are incorrect because the ligand prefix is now 'sulfido' or
'sulfanediido' [thus giving 'trisulfidocarbonate(2−)', 'tetrasulfidophosphate(3−)', etc.]. Section
IU
P-65.2.1.2 of Ref. 1 prescribes 'carbonotrithioic acid', leading to the anion name
'carbonotrithioate', which will not be mistaken for an additive name.
Page 11 of 12
A few examples of other functional nomenclature are also included in Table IR-8.2 (e.g.
'phosphoryl chloride', 'sulfuric diamide'). These particular names are well entrenched and can
still be used, but this type of nomenclature is not recommended for other compounds than
those shown. Again, additive and substitutive names may always be constructed, as
exemplified in the Table.
DRAFT 2 April 2004
12
3.
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
4.
New Blue Book.
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific
Publications, Oxford, 1990.
Nomenclature of Inorganic Chemistry II, Recommendations 2000, Royal Society of
Chemistry, 2001.
IUPAC Nomenclature of Inorganic Chemistry, Second Edition, Definitive Rules 1970,
Butterworths, London, 1971.
tio
n
1.
2.
s
REFERENCES
da
IR-8.7
DRAFT 2 April 2004
Page 12 of 12
1
Table IR-8.1 Acceptable common names and fully systematic (additive) names for oxoacid and related structures. (March 2004)
s
n
This Table includes compounds containing oxygen and hydrogen and at least one other element and with at least one OH group; certain isomers; and examples
of corresponding partially and fully dehydronated anions. Formulae are given in the classical oxoacid format with the 'acid' (oxygen-bound) hydrogens listed
first, followed by the central atom(s), then the hydrogen atoms bound directly to the central atom, and then the oxygen atoms (e.g. HBH2O, H2P2H2O5), except
o
i
at
d
n
e
m
m
o
c
for chain compounds such as e.g. HOCN. In most cases formulae are also written as for coordination entities, assembled according to the principles of Chapter
IR-7 (e.g. the Table gives 'HBH2O = [BH2(OH)]' and 'H2SO4 = [SO2(OH)2]'). More names of oxoanions are given in Table IX.
Note that Section P-42 of Ref. 1 lists a great many inorganic oxoacid structures for use as parent structures in the naming of organic derivatives, cf. the
discussion in Section IR-8.1. Most of those structures, but not all, are included here; a number of di- and polynuclear acids are not explicitly included.
Formula
Common name
(unless otherwise stated)
H3BO3 = [B(OH)3]
H2BO3− = [BO(OH)2]−
HBO32− = [BO2(OH)]2−
[BO3]3−
(HBO2)n = (B(OH)O)n
(BO2−)n = (OBO)n n−
H2BHO2 = [BH(OH)2]
HBH2O = [BH2(OH)]
C
A
P
U
boric acid a
dihydrogenborate
hydrogenborate
borate
metaboric acid
metaborate
boronic acid
borinic acid
l
a
n
io
s
i
v
o
r
P
Fully systematic additive name(s)
e
R
trihydroxidoboron
dihydroxidooxidoborate(1−)
hydroxidodioxidoborate(2−)
trioxidoborate(3−)
catena-poly[hydroxidoboron-µ-oxido]
catena-poly[(oxidoborate-µ-oxido)(1−)]
hydridodihydroxidoboron
dihydridohydroxidoboron
H2CO3 = [CO(OH)2]
HCO3− = [CO2(OH)]−
[CO3]2−
carbonic acid
hydrogencarbonate
carbonate
dihydroxidooxidocarbon
hydroxidodioxidocarbonate(1−)
trioxidocarbonate(2−)
HOCN = [C(N)OH]
HNCO = [C(NH)O]
cyanic acid
isocyanic acid
hydroxidonitridocarbon
oxidoazanediidocarbon, (hydridonitrato)oxidocarbon
I
Page 1 of 8
DRAFT 2 April 2004
2
OCN− = [C(N)O]−
cyanate
nitridooxidocarbonate(1−)
HONC = [N(C)OH]
(iso)fulminic acid b
carbidohydroxidonitrogen
HCNO = [C(H)NO]
ONC− = [N(C)O]−
(iso)fulminic acid,
formonitrile-N-oxide b
fulminate
hydridonitrosyl-κN-carbon, hydrido(oxidonitrato-N)carbon
carbidooxidonitrate(1−)
H4SiO4 = [Si(OH)4]
[SiO4]4−
(H2SiO3)n = (Si(OH)2O)n
(SiO3)n 2n−
H2Si2O7 = [(HO)Si(O)2OSi(O)2(OH)]
[Si2O7]6− = [O3SiOSiO3]6−
silicic acid a
silicate
metasilicic acid
metasilicate
disilicic acid c
disilicate
tetrahydroxidosilicon
tetraoxidosilicate(4−)
catena-poly[dihydroxidosilicon-µ-oxido]
catena-poly[dioxidosilicate-µ-oxido(1−)]
µ-oxido-bis(hydroxidodioxidosilicon)
µ-oxido-bis(trioxidosilicate)(6−)
H2NO3+ = [NO(OH)2]+
HNO3 = [NO2(OH)]
[NO3]−
H2NHO = [NH2OH]
H2NHO3 = [NHO(OH)2]
HNO2 = [NO(OH)]
[NO2]−
HNH2O2 = [NH2O(OH)]
H2N2O2 = [HON=NOH]
d
C
A
P
U
HN2O2− = [HON=NO]−
I
[N2O2]2− = [ON=NO]2−
nitric acid
nitrate
hydroxylamine e
azonic acid
nitrous acid
nitrite
azinic acid
diazenediol f
o
i
at
d
n
e
m
m
o
c
l
a
n
io
s
i
v
o
r
P
2-hydroxydiazene-1-olate f
diazenediolate f
s
n
e
R
dihydroxidooxidonitrogen(1+), trihydrogen(trioxidonitrate)(1+)
hydroxidodioxidonitrogen
trioxidonitrate(1-)
dihydridohydroxidonitrogen
hydridodihydroxidooxidonitrogen
hydroxidooxidonitrogen
dioxidonitrate(1−)
dihydridohydroxidooxidonitrogen
dihydroxido-1κO,2κO-dinitrogen(N_N), or
1,4-dihydrido-2,3-diazy-1,4-dioxy-[4]catena
hydroxido-1κO-oxido-2κO-dinitrate(N_N)(1−), or
1-hydrido-2,3-diazy-1,4-dioxy-[4]catenate(1−)
dioxido-1κO, 2κO-dinitrate(N_N)(2−), or
DRAFT 2 April 2004
Page 2 of 8
3
2,3-diazy-1,4-dioxy-[4]catenate(2−)
H3PO4 = [PO(OH)3]
phosphoric acid a
H2PO4− = [PO2(OH)2]−
dihydrogenphosphate
2
−
2
−
HPO4 = [PO3(OH)]
hydrogenphosphate
[PO4]3−
phosphate
H2PHO3 = [PHO(OH)2]
phosphonic acid g
[PHO2(OH)]−
hydrogenphosphonate
2
−
[PHO3]
phosphonate
H3PO3 = [P(OH)3]
phosphorous acid g
H2PO3− = [PO(OH)2]−
dihydrogenphosphite
HPO32− = [PO2(OH)]2−
hydrogenphosphite
3
−
[PO3]
phosphite
HPO2 = [P(O)OH]
hydroxyphosphanone h
HPO2 = [P(H)O2]
λ5-phosphanedione h
H2PHO2 = [PH(OH)2]
phosphonous acid
HPH2O2 = [PH2O(OH)]
phosphinic acid
HPH2O = [PH2(OH)]
phosphinous acid
H4P2O7 = [(HO)2P(O)OP(O)(OH)2]
diphosphoric acid c
(HPO3)n = (P(O)(OH)O)n
metaphosphoric acid
H4P2O6 = [(HO)2P(O)P(O)(OH)2]
hypodiphosphoric acid
H2P2H2O5 = [(HO)P(H)(O)OP(H)(O)(OH)]
diphosphonic acid
P2H2O52− = [O2P(H)OP(H)(O)2]2−
diphosphonate
H 3P 3O 9
cyclo-triphosphoric acid
d
n
e
m
m
o
c
l
a
s
i
v
H5P3O10
Page 3 of 8
I
s
n
o
i
at
n
io
C
A
P
U
trihydroxidooxidophosphorus
dihydroxidodioxidophosphate(1−)
hydroxidotrioxidophosphate(2−)
tetraoxidophosphate(3−)
hydridodihydroxidooxidophosphorus
hydridohydroxidodioxidophosphate(1−)
hydridotrioxidophosphate(2−)
trihydroxidophosphorus
dihydroxidooxidophosphate(1−)
hydroxidodioxidophosphate(2−)
trioxidophosphate(3−)
hydroxidooxidophosphorus
hydroxidooxidophosphorus
hydridodihydroxidophosphorus
dihydridohydroxidooxidophosphorus
dihydridohydroxidophosphorus
µ-oxido-bis(dihydroxidooxidophosphorus)
catena-poly[hydroxidooxidophosphorus-µ-oxido]
bis[dihydroxidooxidophosphorus](P_P)
o
r
P
e
R
µ-oxido-bis(hydridohydroxidooxidophosphorus)
µ-oxido-bis(hydridodioxidophosphate)(2−)
tri-µ-oxido-tris[hydroxidooxidophosphorus], or
catena-triphosphoric acid,
2,4,6-trihydroxido-2,4,6-trioxido-1,3,5-trioxy-2,4,6-triphosphy-[6]cycle
pentahydroxido-1κ2O,2κO,3κ2O-di-µ-oxido-trioxido-
DRAFT 2 April 2004
4
triphosphoric acid c
1κO,2κO,3κO-triphosphorus, or
µ-[hydroxidotrioxidophosphato(2−)-1κO,2κO]-
s
n
o
i
at
H3AsO4 = [AsO(OH)3]
H2AsHO3 = [AsHO(OH)2]
H3AsO3 = [As(OH)3]
H2AsHO2 = [AsH(OH)2]
HAsH2O2 = [AsH2O(OH)]
HAsH2O = [AsH2(OH)]
arsenic acid, arsoric acid i
arsonic acid
arsenous acid, arsorous acid i
arsonous acid
arsinic acid
arsinous acid
bis(dihydroxidooxidophosphorus), or
1,7-dihydrido-2,4,6-trihydroxido-2,4,6-trioxido1,3,5,7-tetraoxy-2,4,6-triphosphy-[7]catena
trihydroxidooxidoarsenic
hydridodihydroxidooxidoarsenic
trihydroxidoarsenic
hydridodihydroxidoarsenic
dihydridohydroxidooxidoarsenic
dihydridohydroxidoarsenic
H3SbO4 = [SbO(OH)3]
H3SbO3 = [Sb(OH)3]
H2SbHO3 = [SbHO(OH)2]
H2SbHO2 = [SbH(OH)2]
HSbH2O2 = [SbH2O(OH)]
HSbH2O = [SbH2(OH)]
antimonic acid, stiboric acid i
antimonous acid, stiborous acid i
stibonic acid
stibonous acid
stibinic acid
stibinous acid
trihydroxidooxidoantimony
trihydroxidoantimony
hydridodihydroxidooxidoantimony
hydridodihydroxidoantimony
dihydridohydroxidooxidoantimony
dihydridohydroxidoantimony
d
trihydroxidooxidosulfur(1+), trihydrogen(tetraoxidosulfate)(1+)
dihydroxidodioxidosulfur
hydroxidotrioxidosulfate(1−)
tetraoxidosulfate(2−)
hydridohydroxidodioxidosulfur
dihydroxidooxidosulfur
hydroxidodioxidosulfate(1−)
H3SO4+ = [SO(OH)3]+
H2SO4 = [SO2(OH)2]
HSO4− = [SO3(OH)]−
[SO4]2−
C
A
P
U
HSHO3 = [SHO2(OH)]
H2SO3 = [SO(OH)2]
HSO3− = [SO2(OH)]−
I
l
a
n
io
o
r
P
s
i
v
sulfuric acid
hydrogensulfate
sulfate
sulfonic acid j
sulfurous acid
hydrogensulfite
d
n
e
m
m
o
c
e
R
DRAFT 2 April 2004
Page 4 of 8
5
[SO3]2−
HSHO2 = [SHO(OH)]
H2SO2 = [S(OH)2]
[SO2]2−
HSOH = [SH(OH)]
HSO− = [SHO]−
H2S2O7 = [(HO)S(O)2OS(O)2(OH)]
[S2O7]2− = [(O)3SOS(O)3]2−
H2S2O6 = [(HO)(O)2SS(O)2(OH)]
[S2O6]2− = [O3SSO3]2−
H2S3O6 = [(HO)(O)2SSS(O)2(OH)]
H2S4O6 = [(HO)(O)2SSSS(O)2(OH)]
H2S2O5 = [(HO)(O)2SS(O)OH]
[S2O5]2− = [O(O)2SS(O)O]2−
H2S2O4 = [(HO)(O)SS(O)(OH)]
[S2O4]2− = [O2SSO2]2−
C
A
P
U
H2SeO4 = [SeO2(OH)2]
[SeO4]2−
H2SeO3 = [SeHO2(OH)] o
H2SeO3 = [SeO(OH)2] o
[SeO3]2−
I
Page 5 of 8
sulfite
sulfinic acid j
sulfanediol k
sulfanediolate k
trioxidosulfate(2−)
hydridohydroxidooxidosulfur
dihydroxidosulfur
dioxidosulfate(2−)
sulfanol k
hydridohydroxidosulfur
hydridooxidosulfate(1−)
µ-oxido-bis(hydroxidodioxidosulfur)
µ-oxido-bis(trioxidosulfate)(2−)
bis(hydroxidodioxidosulfur)(S_S), or
dithionate
trithionic acid c,m
tetrathionic acid c,m
disulfurous acid n
disulfite n
dithionous acid c,l
selenic acid
selenate
selenonic acid j,o
selenous acid o
selenite
l
a
n
io
s
i
v
o
r
P
dithionite
o
i
at
d
n
e
m
m
o
c
sulfanolate k
disulfuric acid c
disulfate
dithionic acid c,l
s
n
1,4-dihydrido-2,2,3,3-tetraoxido-1,4-dioxy-2,3-disulfy-[4]catena
bis(trioxidosulfate)(S_S)(2−), or
2,2,3,3-tetraoxido-1,4-dioxy-2,3-disulfy-[4]catenate(2−)
1,5-dihydrido-2,2,4,4-tetraoxido-1,5-dioxy-2,3,4-trisulfy-[5]catena
1,6-dihydrido-2,2,5,5-tetraoxido-1,6-dioxy-2,3,4,5-tetrasulfy-[6]catena
dihydroxido-1κO,2κO-trioxido-1κ2O,2κO-disulfur(S_S)
pentaoxido-1κ3O,2κ2O-disulfate(S_S)(2−)
bis(hydroxidooxidosulfur)(S_S), or
e
R
1,4-dihydrido-2,3-dioxido-1,4-dioxy-2,3-disulfy-[4]catena
bis(dioxidosulfate)(S_S)(2−), or
2,3-dioxido-1,4-dioxy-2,3-disulfy-[4]catenate(2−)
dihydroxidodioxidoselenium
tetraoxidoselenate(2−)
hydridohydroxidodioxidoselenium
dihydroxidooxidoselenium
trioxidoselenate(2−)
DRAFT 2 April 2004
6
HSeHO2 = [SeHO(OH)]
seleninic acid j
hydridohydroxidooxidoselenium
H6TeO6 = [Te(OH)6]
[TeO6]6−
H2TeO4 = [TeO2(OH)2]
[TeO4]2−
orthotelluric acid a
orthotellurate a
telluric acid a
tellurate a
tellurous acid
telluronic acid j
tellurinic acid j
hexahydroxidotellurium
hexaoxidotellurate(6−)
dihydroxidodioxidotellurium
tetraoxidotellurate(2−)
dihydroxidooxidotellurium
hydridohydroxidodioxidotellurium
hydridohydroxidooxidotellurium
HClO2 = [ClO(OH)]
[ClO2]−
perchloric acid
perchlorate
chloric acid
chlorate
chlorous acid
chlorite
hydroxidotrioxidochlorine
tetraoxidochlorate(1−)
hydroxidodioxidochlorine
trioxidochlorate(1−)
hydroxidooxidochlorine
dioxidochlorate(1−)
HClO = [ClOH]
[ClO]−
hypochlorous acid
hypochlorite
H2TeO3 = [TeO(OH)2]
HTeHO3 = [TeHO2(OH)]
HTeHO2 = [TeHO(OH)]
HClO4 = [ClO3(OH)]
[ClO4]−
HClO3 = [ClO2(OH)]
[ClO3]−
HBrO4 = [BrO3(OH)]
[BrO4]−
HBrO3 = [BrO2(OH)]
[BrO3]−
HBrO2 = [BrO(OH)]
[BrO2]−
HBrO = [BrOH]
I
C
A
P
U
o
i
at
d
n
e
m
m
o
c
l
a
n
io
s
i
v
o
r
P
s
n
e
R
hydroxidochlorine
oxidochlorate(1−)
perbromic acid
perbromate
bromic acid
bromate
bromous acid
bromite
hydroxidotrioxidobromine
tetraoxidobromate(1−)
hydroxidodioxidobromine
trioxidobromate(1−)
hydroxidooxidobromine
dioxidobromate(1−)
hypobromous acid
hydroxidobromine
DRAFT 2 April 2004
Page 6 of 8
7
[BrO]−
hypobromite
oxidobromate(1−)
H5IO6 = [IO(OH)5]
[IO6]5−
HIO4 = [IO3(OH)]
[IO4]−
HIO3 = [IO2(OH)]
[IO3] −
HIO2 = [IO(OH)]
[IO2]−
orthoperiodic acid a
orthoperiodate a
periodic acid a
periodate a
iodic acid
iodate
iodous acid
iodite
pentahydroxidooxidoiodine
hexaoxidoiodate(5−)
hydroxidotrioxidoiodine
tetraoxidoiodate(1−)
hydroxidodioxidoiodine
trioxidoiodate(1−)
hydroxidooxidoiodine
dioxidoiodate(1−)
HIO = [IOH]
[IO]−
hypoiodous acid
hypoiodite
hydroxidoiodine
oxidoiodate(1−)
s
n
o
i
at
d
n
e
m
m
o
c
l
a
e
R
a The prefix 'ortho' has not been used consistently in the past (including in Chapter I-9 of Ref. 1). Here, it has been removed in the cases of boric acid, silicic acid
n
io
and phosphoric acid where there is no ambiguity in the names without 'ortho'. The only cases where 'ortho' distinguishes between two different compounds are
the telluric and periodic acids (and corresponding anions).
b The names 'fulminic acid' and 'isofulminic acid' have been used inconsistently in the past. The compound originally named fulminic acid is HCNO, which is
not an oxoacid, while the esters usually called 'fulminates' in organic chemistry are RONC, corresponding to the oxoacid HONC. The name 'formonitrile-Noxide' and the additive names in the right hand column specify the structures unambiguously. (See also Table IX under entries for CHNO and CNO).
c The oligomeric series can be continued, e.g. diphosphoric acid, triphosphoric acid, etc.; dithionic acid, trithionic acid, tetrathionic acid, etc.; dithionous,
trithionous, etc.
d The names 'nitric acidium', 'sulfuric acidium', etc. for the hydronated acids represent a hybrid of several nomenclatures and are difficult to translate into certain
languages. They are no longer recommended.
e The substitutive name would be 'azanol'. However, for preferred names for certain organic derivatives, NH2OH itself is regarded as a parent with the name
s
i
v
I
C
A
P
U
o
r
P
'hydroxylamine'. See Ref. 1, Section P-68.3.1.1.
Page 7 of 8
DRAFT 2 April 2004
8
f These are fully systematic substitutive names. The traditional names 'hyponitrous acid' and 'hyponitrite' are not recommended; the systematics otherwise
s
n
adhered to for use of the prefix 'hypo' would have prescribed 'hypodinitrous' and 'hypodinitrite'.
g The name 'phosphorous acid' and the formula H3PO3 have been used in the literature for both [P(OH)3] and [PHO(OH)2]. The present choice of names for
o
i
at
these two structures is in accord with the parent names given in Sections P-42.3 and P-42.4 of Ref. 1.
h These are substitutive names. The situation is similar to that described in footnote g, but no 'acid' names are commonly used for the two isomers of HPO2.
d
n
e
m
m
o
c
i The names 'arsoric', 'arsorous', 'stiboric' and 'stiborous' are included because they are used as parent names in Ref. 1 (Section P-42.4).
j Caution is needed if using the names 'sulfonic acid', 'sulfinic acid',
'selenonic acid', etc. for these compounds. Substitutive nomenclature prescribes using
substitution into parent hydrides rather than into the acids when naming corresponding functional derivatives, e.g. 'trisulfanedisulfonic acid' (not
'trisulfanediyl...'), cf. footnote m; 'methaneseleninic acid' (not methyl-'); etc. Note that the substituent groups 'sulfonyl', 'sulfinyl', etc., are −S(O)2−, −S(O)−,
etc., not HS(O)<, HS(O)−, etc.
k These are fully systematic substitutive names. Names based on the traditional names 'sulfoxylic acid' for S(OH)2 and 'sulfenic acid' for HSOH, and indeed
these names themselves, are no longer recommended.
e
R
l Systematic use of the prefix 'hypo' would give the names 'hypodisulfuric acid' for dithionic acid and 'hypodisulfurous acid ' for dithionous acid.
l
a
m The homologues trithionic acid, tetrathionic acid, etc., may be alternatively named by substitutive nomenclature as 'sulfanedisulfonic acid', 'disulfanedisulfonic
n
io
acid', etc.
n This common name presents a problem because the unsymmetrical structure is not the structure which would otherwise be associated with the 'diacid'
construction ('disulfurous acid' would systematically be [HO(O)SOS(O)OH]). The use of an additive name eliminates this potential confusion, but the problem
with the use of 'disulfurous acid' as a parent name persists in the naming of organic derivatives.
o The formula H2SeO3 has been used in the literature for both selenonic acid and selenous acid. The present choice of names for the two structures shown is in
s
i
v
o
r
P
accord with the parent names given in Sections P-42.1 and P-42.4 of Ref. 1.
I
C
A
P
U
DRAFT 2 April 2004
Page 8 of 8
1
Table IR-8.2 Common names, functional replacement names, and fully systematic (additive) names for some functional
replacement derivatives of oxoacids. (Draft march 2004)
s
n
o
i
at
This Table gives acceptable common names, functional replacement names (cf. Section IR-8.6) and fully systematic (additive) names for
compounds related to oxoacids in Table IR-8.1 and certain isomers and corresponding anions. The examples given are derived by formal
replacement of an O atom/O atoms, or of an OH group/OH groups, by (an)other atom(s) or group(s).
d
n
e
m
m
o
c
Formulae are in some cases given in the classical format with the 'acid' (oxygen- or chalcogen-bound) hydrogen atoms listed first (e.g.
H2S2O3). In most cases formulae are also (or only) written as coordination entities, assembled according to the principles of Chapter IR-7
(e.g. 'H2S2O3 = [SO(OH)2S]').
Formula
Common name
HNO4 = [NO2(OOH)]
NO4− = [NO2(OO)]−
peroxynitric acid a
peroxynitrate a
[NO(OOH)]
[NO(OO)]−
NO2NH2 = N(NH2)O2
l
a
n
io
s
i
v
peroxynitrous acid
a
peroxynitrite
nitramide
C
A
P
U
a
o
r
P
H3PO5 = [PO(OH)2(OOH)] peroxyphosphoric acid a
[PO5]3− = [PO3(OO)]3−
peroxyphosphate a
[PCl3O]
phosphoryl trichloride, or
I
Page 1 of 3
e
R
Functional
replacement name
Fully systematic (additive) name
azoperoxoic acid
azoperoxoate
(dioxidanido)dioxidonitrogen
dioxidoperoxidonitrate(1−)
azoperoxous acid
azoperoxoite
nitric amide
(dioxidanido)oxidonitrogen
oxidoperoxidonitrate(1−)
amidodioxidonitrogen,
dihydrido-1κ2H-dioxido-2κ2O-dinitrogen
phosphoroperoxoic acid
phosphoroperoxoate
phosphoryl trichloride
(dioxidanido)dihydroxidooxidophosphorus
trioxidoperoxidophosphate(3−)
trichloridooxidophosphorus
phosphorus trichloride oxide
DRAFT 2 April 2004
2
H4P2O8 = [(HO)2P(O)OOP(O)(OH)2]
peroxydiphosphoric acid a
[P2O8]4− = [O3POOPO3]4− peroxydiphosphate a
peroxydiphosphoric acid
peroxydiphosphate
µ-peroxido-bis(dihydroxidooxidophosphorus)
µ-peroxido-bis(trioxidophosphate)(4−)
H2SO5 = [SO2(OH)(OOH)] peroxysulfuric acid a
[SO5]2− = [SO3(OO)]2−
peroxysulfate a
sulfuroperoxoic acid
sulfuroperoxoate
(dioxidanido)hydroxidodioxidosulfur
trioxidoperoxidosulfate(2−)
H2S2O8 = [(HO)S(O)2OOS(O)2(OH)]
peroxydisulfuric acid a
[S2O8]2− = [O3SOOSO3]2− peroxydisulfate a
peroxydisulfuric acid
peroxydisulfate
H2S2O3 = [SO(OH)2S]
H2S2O3 = [SO2(OH)(SH)]
S2O32− = [SO3S]2−
H2S2O2 = [S(OH)2S]
H2S2O2 = [SO(OH)(SH)]
[SO2S]2−
SO2Cl2 = [SCl2O2]
thiosulfuric acid
thiosulfuric acid
thiosulfate
thiosulfurous acid
thiosulfurous acid
thiosulfite
sulfuryl dichloride, or
sulfurothioic O-acid
sulfurothioic S-acid
sulfurothioate
sulfurothious O-acid
sulfurothious S-acid
sulfurothioite
sulfuryl dichloride
SOCl2 = [SCl2O]
sulfur dichloride dioxide
thionyl dichloride, or
sulfurous dichloride
dichloridooxidosulfur
sulfur dichloride oxide
sulfamic acid
sulfuric diamide
sulfuramidic acid
sulfuric diamide
amidohydroxidodioxidosulfur
diamidodioxidosulfur
C
A
P
U
[S(NH2)O2(OH)]
[S(NH2)2O2]
I
HSCN = [C(N)(SH)]
HNCS = [C(NH)S]
SCN−
thiocyanic acid
isothiocyanic acid
thiocyanate
o
i
at
d
n
e
m
m
o
c
e
R
n
io
s
i
v
o
r
P
l
a
s
n
µ-peroxido-bis(hydroxidodioxidosulfur)
µ-peroxido-bis(trioxidosulfate)(2−)
dihydroxidooxidosulfidosulfur
hydroxidodioxidosulfanidosulfur
trioxidosulfidosulfate(2−)
dihydroxidosulfidosulfur
hydroxidooxidosulfanidosulfur
dioxidosulfidosulfate(2−)
dichloridodioxidosulfur
nitridosulfanidocarbon
imidosulfidocarbon
nitridosulfidocarbonate(1−)
DRAFT 2 April 2004
Page 2 of 3
3
a
These names were given with the prefix 'peroxo' rather than 'peroxy' in Ref. 4 (Rule 5.22). However, in Ref. 2 names with the prefix 'peroxo'
were dismissed, with no reason given, and no other prefix names were provided instead. The names with the prefix 'peroxy' continue to be in
frequent use. Furthermore, the general rule in functional replacement nomenclature (Ref. 1, Sec. P-15.5.2) is that the replacement prefix for the
replacement −Ο− → −ΟΟ− is, indeed, 'peroxy' (as opposed to the infix for this replacement, which is 'peroxo'). In view of this, the names with
s
n
o
i
at
the prefix 'peroxy' are listed here. For mononuclear oxoacids, the present rules in Ref. 1 (Section P-67.1) prescribe using the infix method; the
resulting names are given here in the second column.
d
n
e
m
m
o
c
l
a
e
R
n
io
s
i
v
I
Page 3 of 3
C
A
P
U
o
r
P
DRAFT 2 April 2004
1
IR-9
Coordination Compounds (Draft March 2004)
tio
n
s
CONTENTS
IR-9.1 Introduction
IR-9.1.1 General
da
IR-9.1.2 Definitions
IR-9.1.2.1 Background
IR-9.1.2.3 Central atom
IR-9.1.2.4 Ligands
om
me
n
IR-9.1.2.2 Coordination compounds and the coordination entity
IR-9.1.2.5 Coordination polyhedron
IR-9.1.2.6 Coordination number
IR-9.1.2.7 Chelation
ec
IR-9.1.2.8 Oxidation state
IR-9.1.2.9 Coordination nomenclature: an additive nomenclature
lR
IR-9.1.2.10 Bridging ligands
IR-9.1.2.11 Metal-metal bonds
na
IR-9.2 Describing the constitution of coordination compounds
IR-9.2.1 General
isi
o
IR-9.2.2 Naming coordination compounds
IR-9.2.2.1 Sequences of ligands and central atoms within names
IR-9.2.2.2 Number of ligands in a coordination entity
ov
IR-9.2.2.3 Representing ligands in names
Pr
IR-9.2.2.4 Charge numbers, oxidation numbers, and ionic proportions
IR-9.2.3 Formulae of coordination compounds
PA
C
IR-9.2.3.1 Sequence of symbols within the coordination formula
IR-9.2.3.2 Use of enclosing marks
IR-9.2.3.3 Ionic charges and oxidation numbers
IR-9.2.3.4 Use of abbreviations
IU
IR-9.2.4 Specifying donor atoms
IR-9.2.4.1 General
IR-9.2.4.2 The kappa convention
IR-9.2.4.3 Comparison of the eta and kappa nomenclatures
Page 1 of 69
DRAFT 2 April 2004
2
IR-9.2.4.4 Donor atom symbol to indicate points of ligation
IR-9.2.5 Polynuclear complexes
s
IR-9.2.5.1 General
tio
n
IR-9.2.5.2 Bridging ligands
IR-9.2.5.3 Metal-metal bonding
IR-9.2.5.5 Unsymmetrical dinuclear entities
om
me
n
IR-9.2.5.6 Trinuclear and larger structures
da
IR-9.2.5.4 Symmetrical dinuclear entities
IR-9.2.5.7 Polynuclear clusters: symmetrical central structural units
IR-9.2.5.8 Polynuclear clusters: unsymmetrical central structural units
IR-9.3 Describing the configuration of coordination compounds
IR-9.3.1 Introduction
IR-9.3.2 Describing the coordination geometry
ec
IR-9.3.2.1 Polyhedral symbol
IR-9.3.2.2 Choosing between closely related geometries
lR
IR-9.3.3 Describing relative configuration – distinguishing between
diastereoisomers
na
IR-9.3.3.1 General
IR-9.3.3.2 Configuration index
isi
o
IR-9.3.3.3 Square planar coordination systems (SP-4)
IR-9.3.3.4 Octahedral coordination systems (OC-6)
IR-9.3.3.5 Square pyramidal coordination systems (SPY-4, SPY-5)
ov
IR-9.3.3.6 Bipyramidal coordination systems (TBPY-5, PBPY-7, HBPY-8
and HBPY-9)
Pr
IR-9.3.3.7 T-shaped systems (TS-3)
IR-9.3.3.8 See-saw systems (SS-4)
IU
PA
C
IR-9.3.4 Describing absolute configuration – distinguishing between
enantiomers
IR-9.3.4.1 General
IR-9.3.4.2 The R/S convention for tetrahedral centres
IR-9.3.4.3 The R/S convention for trigonal bipyramidal centres
IR-9.3.4.4 The C/A convention for other polyhedral centres
IR-9.3.4.5 The C/A convention for trigonal bipyramidal centres
IR-9.3.4.6 The C/A convention for square pyramidal centres
DRAFT 2 April 2004
Page 2 of 69
3
IR-9.3.4.7 The C/A convention for see-saw centres
IR-9.3.4.8 The C/A convention for octahedral centres
s
IR-9.3.4.9 The C/A convention for trigonal prismatic centres
tio
n
IR-9.3.4.10 The C/A convention for other bipyramidal centres
IR-9.3.4.11 The skew-lines convention
da
IR-9.3.4.12 Application of the skew-lines convention to tris(bidentate)
octahedral complexes
om
me
n
IR-9.3.4.13 Application of the skew-lines convention to bis(bidentate)
octahedral complexes
IR-9.3.4.14 Application of the skew-lines convention to conformations of
chelate rings
IR-9.3.5 Determining ligand priority
IR-9.3.5.1 General
IR-9.3.5.2 Priority numbers
ec
IR-9.3.5.3 Priming convention
INTRODUCTION
IR-9.1.1
General
isi
o
na
IR-9.1
lR
IR-9.4 Final remarks
IR-9.5 References
Pr
ov
This Chapter presents the definitions and rules necessary for formulating and naming
coordination compounds. Key terms such as coordination entity, coordination polyhedron,
coordination number, chelation and bridging ligands are first defined and the role of additive
nomenclature explained (see also Chapter IR-7).
IU
PA
C
These definitions are then used to develop rules for writing the names and formulae of
coordination compounds. The rules allow the composition of coordination compounds to be
described in a way that is as unambiguous as possible. The names and formulae provide
information about the nature of the central atom, the ligands that are attached to it, and the
overall charge on the structure.
Page 3 of 69
Stereochemical descriptors are then introduced as a means of identifying or distinguishing
between the diastereoisomeric or enantiomeric structures that may exist for a compound of
any particular composition.
DRAFT 2 April 2004
4
IR-9.1.2
Definitions
IR-9.1.2.1
Background
om
me
n
da
tio
n
s
The description of the configuration of a coordination compound requires first that the
coordination geometry be specified using a polyhedral symbol (Section IR-9.3.2.1). Once
this is done the relative positions of the ligands around the coordination polyhedron are
specified using the configuration index (Section IR-9.3.3). The configuration index is a
sequence of ligand priority numbers produced by following rules specific to each
coordination geometry. If required, the chirality of a coordination compound can be
described, again using ligand priority numbers (Section IR-9.3.4). The ligand priority
numbers used in these descriptions are based on the chemical composition of the ligands. A
detailed description of the rules by which they are obtained is provided in Section P-91 of
Ref. 1, but an outline is given in Section IR-9.3.5.
lR
ec
The development of coordination theory and the identification of a class of compounds called
coordination compounds began with the historically significant concepts of primary and
secondary valence.
isi
o
na
Primary valences were obvious from the stoichiometries of simple compounds such as
NiCl2, Fe2(SO4)3, and PtCl2. However, new materials were frequently observed when other,
independently stable substances, e.g. H2O, NH3, and KCl, were added to these simple
compounds giving, for example, NiCl2.4H2O, Co2(SO4)3.12NH3 or PtCl2.2KCl. Such
Pr
ov
species were called complex compounds, in recognition of the stoichiometric complications
they represented, and were considered characteristic of certain metallic elements. The number
of species considered to be added to the simple compounds gave rise to the concept of
secondary valence.
IU
PA
C
Recognition of the relationships between these complex compounds led to the formulation of
coordination theory and the naming of coordination compounds using additive nomenclature.
Each coordination compound either is, or contains, a coordination entity (or complex) that
consists of a central atom to which other groups are bound.
While these concepts have usually been applied to metal compounds, a wide range of other
species can be considered to consist of a central atom to which a number of other groups are
bound. The application of additive nomenclature to such species is described in Chapter 7.
DRAFT 2 April 2004
Page 4 of 69
5
IR-9.1.2.2
Coordination compounds and the coordination entity
Examples:
1.
2.
3.
[Co(NH3)6]3+
_
[PtCl4]2
[Fe3(CO)12]
Central atom
ec
IR-9.1.2.3
om
me
n
da
tio
n
s
A coordination compound is any compound that contains a coordination entity. A
coordination entity is an ion or neutral molecule that is composed of a central atom, usually
that of a metal, to which is attached a surrounding array of other atoms or groups of atoms,
each of which is called a ligand. Classically, a ligand was said to satisfy either a secondary or
a primary valence of the central atom and the sum of these valences (often equal to the
number of ligands) was called the coordination number (see Section I-9.1.2.6). In formulae,
the coordination entity is enclosed in square brackets whether it is charged or uncharged (see
Section I-9.2.3.2).
na
lR
The central atom is the atom in a coordination entity which binds other atoms or groups of
atoms (ligands) to itself, thereby occupying a central position in the coordination entity. The
central atoms in [NiCl2(H2O)4], [Co(NH3)6]3+ and [PtCl4]2- are nickel, cobalt and platinum,
IR-9.1.2.4
Ligands
isi
o
respectively.
IR-9.1.2.5
Pr
ov
The ligands are the atoms or groups of atoms bound to the central atom. The root of the
word is often converted into other forms, such as to ligate, meaning to coordinate as a ligand,
and the derived participles, ligating and ligated.
Coordination polyhedron
IU
PA
C
It is standard practice to regard the ligand atoms directly attached to the central atom as
defining a coordination polyhedron (or polygon) about the central atom. Thus [Co(NH3)6]3+
_
is an octahedral ion and [PtCl4]2 is a square planar ion. In this way the coordination
number may equal the number of vertices in the coordination polyhedron. This definition
does not necessarily apply to organometallic compounds, where more than one atom of the
ligand may be involved in a single bond to the central atom (see Chapter IR-10).
Examples:
Page 5 of 69
DRAFT 2 April 2004
6
B
B
B
A
B
B
B
1. octahedral
coordination
polyhedron
B
B
2. square planar
coordination
polygon
3. tetrahedral
coordination
polyhedron
om
me
n
Coordination number
B
da
B
A
tio
n
A
IR-9.1.2.6
B
B
s
B
B
For coordination compounds, the coordination number equals the number of σ-bonds
between ligands and the central atom. Note that where both σ- and π-bonding occurs
_
between the ligating atom and the central atom, e.g. with ligands such as CN , CO, N2 and
PMe3, the π-bonds are not considered in determining the coordination number.
Chelation
ec
IR-9.1.2.7
lR
Chelation involves coordination of more than one σ-electron pair donor atom from the same
na
ligand to the same central atom. The number of such ligating atoms in a single chelating
ligand is indicated by the adjectives bidentate,2 tridentate, tetradentate, pentadentate, etc. (see
Examples:
isi
o
Table IV* for a list of numerical prefixes). The number of donor atoms from a given ligand
attached to the same central atom is called the denticity.
CH2
ov
H2C
H 2N
NH2
H 2C
H 2N
Pr
Pt
NH
CH2CH2NH2
Pt
Cl
1. bidentate chelation
Cl
Cl
2. bidentate chelation
IU
PA
C
Cl
CH2
*
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
Page 6 of 69
7
H 2N
NH
Pt
Cl
N
H2
CH2
H 2C
CH2
H 2C
CH2
HN
NH
Pt
N
H2
2+
CH2
N
H2
CH2
4. tetradentate chelation
da
3. tridentate chelation
H 2C
s
+
CH2
tio
n
H 2C
om
me
n
The cyclic structures formed when more than one donor atom from the same ligand is bound
to the central atom are called chelate rings, and the process of coordination of these donor
atoms is called chelation.
[(H3N)5Co(µ-NH2CH2CH2NH2)Co(NH3)5]6+
lR
Example:
1.
ec
If a potentially bidentate ligand, such as ethane-1,2-diamine, coordinates to two metal ions, it
does NOT chelate but coordinates in a monodentate fashion to each metal ion, forming a
connecting link or bridge.
Oxidation state
ov
IR-9.1.2.8
isi
o
na
Alkenes, arenes and other unsaturated molecules attach to central atoms, using some or all of
their multiply bonded atoms, to give organometallic complexes. While there are many
similarities between the nomenclature of coordination and organometallic compounds, the
latter differ from the former in clearly definable ways. Organometallic complexes are
therefore treated separately in Chapter IR-10.
IU
PA
C
Pr
The oxidation state of a central atom in a coordination entity is defined as the charge it would
bear if all the ligands were removed along with the electron pairs that were shared with the
central atom. It is represented by a Roman numeral.
Page 7 of 69
The general and systematic treatment of oxidation state follows from the application of the
classical definition of coordination number. It must be emphasized that oxidation state is an
index derived from a simple and formal set of rules (see also Sections IR-4.6.1 and IR5.4.2.2) and that it is not a direct indicator of electron distribution. In certain cases, the
formalism does not give acceptable central atom oxidation states. In such ambiguous cases,
the net charge on the coordination entity is preferred in most nomenclature practices. The
following examples illustrate the relationship between oxidation state and coordination
number.
DRAFT 2 April 2004
8
4.
[MnFO3]
6 NH3
_
4 Cl
_
4 O2
_
_
3 O2 + 1 F
_
_
5 CN + 1 H
2.
5.
IR-9.1.2.9
[Co(CN)5H]
_
[Fe(CO)4]2
4 CO
VII
VII
III
-II
om
me
n
6.
3_
II
s
3.
[Co(NH3)6]3+
_
[CoCl4]2
_
[MnO4]
1.
Central atom
oxidation state
III
tio
n
Ligands
da
Formula
Coordination nomenclature: an additive nomenclature
ec
When coordination theory was first developed, coordination compounds were considered to
be formed by addition of independently stable compounds to a simple central compound.
They were therefore named on the basis of an additive principle, where the names of the
added compounds and the central simple compound were combined. This principle remains
the basis for naming coordination compounds.
lR
The name is built up around the central atom name, just as the coordination entity is built up
Example:
1.
na
around the central atom.
Addition of ligands to a central atom:
isi
o
Ni2+ + 6H2O
[Ni(OH2)6]2+
ov
Addition of ligand names to a central atom name:
hexaaquanickel(II)
PA
C
Pr
This nomenclature then extends to more complicated structures where central atoms (and
their ligands) are added together to form polynuclear species from mononuclear building
blocks.
IU
IR-9.1.2.10
Bridging ligands
In polynuclear species a ligand can also act as a bridging group, by forming bonds to two or
more central atoms simultaneously. Bridging is indicated in names and formulae by adding
the symbol µ as a prefix to the ligand formula or name (see Section IR-9.2.5.2).
DRAFT 2 April 2004
Page 8 of 69
9
tio
n
s
Bridging ligands link central atoms together to produce coordination entities having more
than one central atom. The number of central atoms joined into a single coordination entity
by bridging ligands or metal-metal bonds is indicated by using the terms dinuclear, trinuclear,
tetranuclear, etc.
Examples:
1.
om
me
n
da
The bridge index is the number of central atoms linked by a particular bridging ligand (see
Section IR-9.2.5.2). Bridging can be through one atom or through a longer array of atoms.
Cl
Cl
Al
Cl
Al
Cl
Cl
Cl
ec
Al2(µ-Cl)2Cl4 or Cl2Al(µ-Cl)2AlCl2
lR
2.
6+
H
O
Co(NH3)4
O
H
3
[Co{(µ-OH)2Co(NH3)4}3]6+
Metal-metal bonds
ov
IR-9.1.2.11
isi
o
na
Co
PA
C
Pr
Simple structures that contain a metal-metal bond are readily described using additive
nomenclature (see Section IR-9.2.5.3), but complications arise for structures that involve
three or more central atoms. Species that contain such clusters of central atoms are treated in
Sections IR-9.2.5.6 to IR-9.2.5.8.
IU
Examples:
1.
[Br4ReReBr4]2+
bis(tetrabromidorhenium)(Re—Re)
2.
1 2
Page 9 of 69
DRAFT 2 April 2004
10
s
[(OC)5ReCo(CO)4]
nonacarbonyl-lκ5C,2κ4C-rheniumcobalt(Re—Co)
DESCRIBING THE CONSTITUTION OF COORDINATION COMPOUNDS
IR-9.2.1
General
tio
n
IR-9.2
om
me
n
da
Three main methods are available for describing the constitution of compounds; one can
draw structures, write names or write formulae. A drawn structure contains information about
the structural components of the molecule as well as their stereochemical relationships.
Unfortunately, such structures are not usually suitable for inclusion in text. Names and
formulae are therefore used to describe the chemical composition of a compound.
ec
The name of a coordination compound provides detailed information about the structural components
present. However, it is important that the name can be easily interpreted unambiguously. For that reason,
there should be rules that define how the name is constructed. The following sections detail these rules and
provide examples of their use.
IR-9.2.2
isi
o
na
lR
The name of a compound can, however, be rather long and its use may be inconvenient. In such
circumstances a formula provides a shorthand method of representing the compound. Rules are provided in
order to make the use of formulae more straightforward. It should be noted that, because of their abbreviated
form, it is often not possible to provide as much information about the structure of a compound in its
formula as can be provided by its name.
Naming coordination compounds
PA
C
Pr
ov
The names of coordination compounds are derived by following the principles of additive
nomenclature, as outlined in Chapter IR-7. Thus, the groups that surround the central atom or
structure must be identified in the name. They are listed as prefixes to the name of the central
atom (see Section IR-9.2.2.1) along with any appropriate multipliers (see Section IR-9.2.2.2).
These prefixes are usually derived in a simple way from the ligand name (see Section IR9.2.2.3).
IR-9.2.2.1
Sequences of ligands and central atoms within names
IU
The following rules are used when naming coordination compounds:
(i)
(ii)
ligand names are listed before the name of the central atom,
no spaces are left between parts of the name that refer to the same coordination entity,
DRAFT 2 April 2004
Page 10 of 69
11
s
(iv)
ligand names are listed in alphabetical order (numerical prefixes indicating the
number of ligands are not considered in determining that order),
the use of abbreviations in names is discouraged.
tio
n
(iii)
Example:
1.
[CoCl(NH3)5]Cl2
[AuXe4]2+
tetraxenonidogold(2+)
IR-9.2.2.2
om
me
n
2.
da
pentaamminechloridocobalt(2+) chloride
Number of ligands in a coordination entity
Two kinds of numerical prefix are available for indicating the number of each type of ligand
within the name of the coordination entity (see Table IV).
na
lR
(ii)
Prefixes di, tri, etc. are generally used for simple ligands. Enclosing marks are not
required.
Prefixes bis, tris, tetrakis, etc. are used with complex expressions and when required
to avoid ambiguity. Enclosing marks (the nesting order of which is described in
Section IR-2.2) must be placed around the multiplicand.
ec
(i)
For example, one would use diammine, for (NH3)2, but bis(methylamine), for (NH2Me)2, to
Representing ligands in names
ov
IR-9.2.2.3
isi
o
make a distinction from dimethylamine. (Note that this ambiguity does not arise if the
preferred IUPAC name1, methanamine, is used instead of methylamine). There is no elision
of vowels or use of a hyphen, e.g. in tetraammine and similar names.
PA
C
Pr
Systematic and alternative names for some common ligands are given in Table IX. The general
features are as follows:
IU
(i)
Page 11 of 69
Names of anionic ligands, whether inorganic or organic, are modified to end in 'o'
(see Section IR-7.1.3). In general, if the anion name ends in 'ide', 'ite' or 'ate', the final
'e' is replaced by 'o', giving 'ido', 'ito', and 'ato', respectively. In particular, alcoholates,
thiolates, phenolates, carboxylates, partially dehydronated amines, phosphanes, etc.
are in this category.3
In its complexes, hydrogen is always treated as anionic. 'Hydrido' is used for
hydrogen coordinating to all elements including boron.4
DRAFT 2 April 2004
12
Names of neutral and cationic ligand names, including organic ligands,5 are used
without modification (even if they carry the endings 'ide', 'ite' or 'ate').
(iii)
Enclosing marks are required for neutral and cationic ligand names, for inorganic
anionic ligands containing numerical prefixes (such as triphosphato), for any
compositional name (such as carbon disulfide), for any substituted organic ligand
(even if there is no ambiguity in its use), and wherever necessary to avoid ambiguity.
However, common ligands such as aqua, ammine, carbonyl, nitrosyl, methyl, ethyl,
etc., do not require enclosing marks, unless there is ambiguity.
(iv)
Ligands binding to metals through carbon atoms are treated in Chapter IR-10, on
organometallic compounds.
om
me
n
da
tio
n
s
(ii)
Examples:
Formula
Ligand name
3.
4.
cyanido3
hydrido
deuterido or [2H]hydrido
_
PhCH2CH2Se
2-phenylethaneselanolato
_
MeCOO
acetato or ethanoato
_
Me2N
dimethylazanido or dimethylamido
MeCONH2
acetamide (not acetamido)
_
MeCONH
acetylazanido or acetylamido (not acetamido)
MeNH2
methanamine
_
MeNH
methylazanido or methanaminido
(anion itself named methanaminide, see Section P-72.2.2.3 of Ref. 1)
6.
7.
8.
ov
9.
10.
isi
o
na
5.
ec
2.
chlorido3
lR
_
Cl
_
CN
_
H
_
_
D or 2H
1.
Pr
11.
12.
PA
C
13.
14.
15.
IU
IR-9.2.2.4
MePH2
_
MePH
MeOS(O)OH
_
MeOS(O)O
methylphosphane
methylphosphanido
methyl hydrogen sulfite
methyl sulfito, or methoxidodioxidosulfato(1-)
Charge numbers, oxidation numbers, and ionic proportions
The following methods can be used to assist in describing the composition of a compound:
DRAFT 2 April 2004
Page 12 of 69
13
s
(i)
All anionic coordination entities take the ending 'ate', whereas no distinguishing termination is used
for cationic or neutral coordination entities.
da
tio
n
(ii)
The oxidation number of the central atom may be indicated by appending a Roman numeral to the
central atom name, but only if the oxidation state can be defined without ambiguity. The oxidation number is
enclosed in parentheses after the part of the name denoting the central atom. When necessary a negative sign
is placed before the number. Arabic zero indicates the zero oxidation number.
(iv)
om
me
n
(iii)
Alternatively, the charge on a coordination entity may be indicated. The net charge is written in arabic
numbers, with the number preceding the charge sign, and enclosed in parentheses. It follows the name of the
central atom without the intervention of a space.
The proportions of ionic entities may be given by using stoichiometric prefixes on both ions.
Examples:
1.
K4[Fe(CN)6]
3.
hexaamminecobalt(III) chloride
[CoCl(NH3)5]Cl2
na
lR
ec
2.
potassium hexacyanidoferrate(II), or
potassium hexacyanidoferrate(4_), or
tetrapotassium hexacyanidoferrate
[Co(NH3)6]Cl3
5.
pentaamminechloridocobalt(2+) chloride
[CoCl(NH3)4(NO2)]Cl
tetraamminechloridonitrito-κN-cobalt(III) chloride
[PtCl(NH2Me)(NH3)2]Cl
6.
diamminechlorido(methanamine)platinum(II) chloride
[CuCl2{O=C(NH2)2}2]
ov
dichloridobis(urea)copper(II)
K2[PdCl4]
Pr
7.
isi
o
4.
8.
11.
hexakis(methyl isocyanide)iron(II) bromide
[Co(en)3]Cl3
PA
C
10.
potassium pentachloridonitridoosmate(2_)
Na[PtBrCl(NH3)(NO2)]
sodium amminebromidochloridonitrito-κN-platinate(1_)
[Fe(CNMe)6]Br2
IU
9.
Page 13 of 69
potassium tetrachloridopalladate(II)
K2[OsCl5N]
tris(ethane-1,2-diamine)cobalt(III) trichloride
DRAFT 2 April 2004
14
IR-9.2.3
Formulae of coordination compounds
Sequence of symbols within the coordination formula
om
me
n
IR-9.2.3.1
da
tio
n
s
The formula of a compound is a shorthand tool used to provide basic information about the
constitution of the compound in a concise and convenient manner. Different applications may require
flexibility in the writing of formulae. Thus, on occasion, it may be desirable to violate the following
guidelines in order to provide more information about the structure of the compound that the formula
represents.
(i)
The central atom is listed first.
(ii)
The ligands are then listed in alphabetical order6 (see Section IR-4.4.2.2), based on
the way the ligand is represented in the formula. Thus, CH3CN, MeCN and NCMe
More information is conveyed by formulae that show ligands with the donor atom
nearest the central atom; this procedure is recommended wherever possible, even for
coordinated water.
(iv)
Ligand linear formulae are sometimes needed.
isi
o
na
(iii)
Use of enclosing marks
ov
IR-9.2.3.2
lR
ec
would be ordered under C, M and N respectively. The placement of the ligand in the
list does not depend on the charge of the ligand. Polydentate ligands are included in
the alphabetical list, according to Section IR-4.4.2.2. If an abbreviation is used in a
formula to represent a ligand (see Section IR-9.2.3.4), the first letter of the
abbreviation is used to determine the position of the ligand in the alphabetical list.
IU
PA
C
Pr
The formula for the entire coordination entity, whether charged or not, is enclosed in square
brackets. When ligands are polyatomic, their formulae are enclosed in parentheses. Ligand
abbreviations are also usually enclosed in parentheses. In the special case of coordination
entities, the nesting order of enclosures is as given in Sections IR-2.2 and IR-4.2.3. Square
brackets are used only to enclose coordination entities, and parentheses and braces are nested
alternately. There should be no space between representations of ionic species within a
coordination formula.
IR-9.2.3.3
Examples 1-11 in Section IR-9.2.2.4 also illustrate the use of enclosing marks in formulae.
Ionic charges and oxidation numbers
DRAFT 2 April 2004
Page 14 of 69
15
tio
n
s
If the formula of a charged coordination entity is to be written without that of the counter-ion,
the charge is indicated outside the square bracket as a right superscript, with the number
before the sign. The oxidation number of a central atom may be represented by a Roman
numeral, which should be placed as a right superscript on the element symbol.
1.
_
[PtCl6]2
2.
[Cr(OH2)6]3+
3.
4.
[CrIII(NCS)4(NH3)2]
[CrIIICl3(OH2)3]
_
_
[Fe II(CO)4]2
IR-9.2.3.4
_
Use of abbreviations
om
me
n
5.
da
Examples:
lR
ec
Abbreviations can be used to represent complicated organic ligands in formulae (although
they should not normally be used in names). When used in formulae they are usually
enclosed in parentheses.
na
Guidelines for the formulation of ligand abbreviations are given in Section IR-4.4.4;
examples of such abbreviations are listed alphabetically in Table VII with diagrams of most
shown in Table VIII.
ov
isi
o
In cases where coordination occurs through one donor atom of a ligand containing more than
one possible donor site, an indication of that donor atom may be desirable. This may be
achieved through use of the kappa convention (see Section IR-9.2.4.2) in which the Greek
lower case kappa (κ) is used to indicate the donor atom. For example, if the glycinate anion
Specifying donor atoms
PA
C
IR-9.2.4
Pr
(gly) coordinates only through the nitrogen, the abbreviation of the ligand would be shown as
gly-κN, as in the complex [M(gly-κN)3X3].
IU
IR-9.2.4.1
Page 15 of 69
General
There is no need to specify the donor atom of a monodentate ligand that has only one atom
able to form a bond with a central atom. However, ambiguity may arise when there is more
than one possible donor atom in a ligand. It is then necessary to specify which donor atom(s)
of a ligand is (are) bound to the central atom. In particular, this must be done in any case
where there are potential donor atoms that are not bound to the central atom or if there is
DRAFT 2 April 2004
16
tio
n
s
more than one central atom. The only exceptions are common ligands that typically
coordinate in a manner that leaves a potential donor atom uncoordinated. Thus, in terms of
nomenclature, carboxylate groups are usually considered to be monodentate unless otherwise
specified, and carbonyl and nitrosyl ligands are presumed to bind in a monodentate fashion,
through the carbon and nitrogen atoms respectively.
om
me
n
da
In general, however, ligands are presumed to coordinate through all of their donor atoms,
unless otherwise specified. The following sections detail the means by which donor atoms are
specified in those systems where not all donor atoms are coordinated. The first method, the
kappa (κ) convention, is general and can be used for systems of great complexity. The
second, use of the donor atom symbol, is appropriate only for relatively simple systems.
ec
These systems may be used in names, but they are not always suitable for use in formulae.
The use of donor atom symbols is possible in the formulae of simple systems, but care must
be taken to avoid ambiguity. The kappa convention is not generally compatible with the use of
abbreviations.
lR
It should be noted that these methods are only appropriate for cases where each bond is
between the central atom and a single donor atom. The eta (η) nomenclature should be used
isi
o
HB
IU
PA
C
Pr
ov
Example:
1.
na
for any cases where the central atom is bonded to contiguous donor atoms (see IR-10.2.5.1).
Most examples of this latter kind are organometallic compounds (Chapter IR-10) but the
example below shows its use for a coordination compound of dioxygen.
IR-9.2.4.2
N
N
N
N
O
Cu
Cu
O
N
N
N
N
N
N
N
N
BH
µ-η2:η2-bonding of dioxygen
The kappa convention
DRAFT 2 April 2004
Page 16 of 69
17
s
The kappa convention is used to indicate the points of ligation, particularly in complex
examples. Single ligating atom attachments of a polyatomic ligand to a coordination centre
are indicated by the italicized element symbol preceded by a Greek kappa, κ. These symbols
tio
n
are placed after the portion of the ligand name that represents the ring, chain or substituent
group in which the ligating atom is found.
om
me
n
da
Monodentate ambidentate ligands provide simple examples, although for these cases the
kappa convention is not significantly more useful than the 'donor atom symbol' convention
(Section IR-9.2.4.4). Nitrogen-bonded NCS is thiocyanato-κN and sulfur-bonded NCS is
thiocyanato-κS. Nitrogen-bonded nitrite is named nitrito-κN and oxygen-bonded nitrite is
named nitrito-κO as in pentaamminenitrito-κO-cobalt(III).
In cases where two or more identical ligands (or parts of a polydentate ligand) are involved, a
superscript is used on κ.
[NiBr2(Me2PCH2CH2PMe2)]
dibromido[ethane-1,2-diylbis(dimethylphosphane)-κ2P]nickel(II)
lR
1.
ec
Example:
isi
o
na
Donor atoms of a particular element may be distinguished by adding a prime or primes, or a
right superscript numeral, to the element symbol. Primes may be sufficient in simple cases.
(See Example 3 below).
ov
Superscript numerals, on the other hand, are based on an appropriate numbering of some or
all of the atoms of the ligand and allow the position of the bond(s) to the central atom to be
specified even in quite complex cases. (See Example 4 below).
PA
C
Pr
Any multiplicative prefixes for complex entities are presumed to operate on the κ symbol as
well. Thus, one uses the partial name '...bis(2-amino-κN-ethyl)... ' and not '...bis(2-aminoκ2N-ethyl)...' in Example 2 below. Examples 2 and 3 use tridentate chelation by the linear
tetraamine ligand N,N'-bis(2-aminoethyl)ethane-1,2-diamine to illustrate these rules.
IU
Examples:
2.
Page 17 of 69
DRAFT 2 April 2004
18
H 2C
+
CH2
Pt
NH
NH2CH2CH2
da
Cl
s
NHCH2CH2
tio
n
H 2N
3.
H 2C
om
me
n
[N,N'-bis(2-amino-κN-ethyl)ethane-1,2-diamine-κN]chloridoplatinum(II)
+
CH2
H 2N
NH
Pt
Cl
NH
CH2
CH2
ec
CH2CH2NH2
lR
[N-(2-amino-κN-ethyl)-N'-(2-aminoethyl)ethane-1,2-diamine-κ2N,N']chloridoplatinum(II)
isi
o
na
Example 2 illustrates how coordination by the two terminal primary amino groups of the
ligand is indicated by placing the kappa index after the functional group name and within the
effect of the 'bis' doubling prefix. The appearance of the simple index κN after the 'ethane-
ov
1,2-diamine' indicates the binding by only one of the two equivalent secondary amino
nitrogen atoms.
PA
C
Pr
Only one of the primary amines is coordinated in Example 3. This is indicated by not using
the doubling prefix 'bis', repeating (2-aminoethyl), and inserting the κ index only in the first
such unit, i.e. (2-amino-κN-ethyl). The involvement of both of the secondary ethane-1,2diamine nitrogen atoms in chelation is indicated by the index κ2N,N'.
IU
Tridentate chelation by the tetrafunctional macrocycle in Example 4 is shown by the kappa
index following the ligand name. The ligand locants are required in order to distinguish this
complex from those where the central atom is bound to other combinations of the four
potential donor atoms.
Example:
4.
DRAFT 2 April 2004
Page 18 of 69
19
12
1
s
S
tio
n
S
MoI3
S
S
4
da
8
om
me
n
triiodido(1,4,8,12-tetrathiacyclopentadecane-κ3S1,4,8)molybdenum, or
triiodido(1,4,8,12-tetrathiacyclopentadecane-κ3S1,S4,S8)molybdenum
ec
Well established modes of chelation of the (ethane-1,2-diyldinitrilo)tetraacetato ligand (edta),
namely bidentate, tetradentate and pentadentate, are illustrated in Examples 5-8. The
multiplicative prefix 'tetra' used in Example 5 cannot be used in Examples 6 and 7 because of
the need to avoid ambiguity about which acetate arms are coordinated to the central atom.
lR
Examples:
5.
na
H 2C
CH2
isi
o
(O 2CCH2)2N
4-
N(CH2CO2)2
PtII
Cl
Cl
IU
PA
C
Pr
6.
ov
dichlorido[(ethane-1,2-diyldinitrilo-κ2N,N')tetraacetato]platinate(4_)
4-
O
C
CH2
O
N
(CH2)2N(CH2CO2)2
PtII
Cl
CH2CO2
Cl
dichlorido[(ethane-1,2-diyldinitrilo-κN)(N-acetato-κO)triacetato]platinate(II)
7.
Page 19 of 69
DRAFT 2 April 2004
20
N
N
C
O
CH2CO2
CH2
PtII
C
O
O
da
O
2-
s
H 2C
CH2
tio
n
O2CCH2
H2C
8.
om
me
n
[(ethane-1,2-diyldinitrilo-κ2N,N')(N,N'-diacetato-κ2O,O')-N,N-diacetato]platinate(2_)
-
OH2
O
C
CH2
O
CH2CO2
N
Co
CH2
N
ec
O
CH2
CH2
lR
C
O
C
O
na
O
CH2
isi
o
aqua[(ethane-1,2-diyldinitrilo-κ2N,N')tetraacetato-κ3O,O',O'']cobaltate(1_)
Pr
ov
A compound of edta in which one amino group is not coordinated while all four acetates are
bound to a single metal ion would bear the ligand name (ethane-1,2-diyldinitriloκN)tetraacetato-κ4O,O',O'',O''' within the name of the complex.
IU
PA
C
The mixed sulfur-oxygen cyclic polyether 1,7,13-trioxa-4,10,16-trithiacyclooctadecane might
chelate to alkali metals only through its oxygen atoms and to second row transition atoms
only through its sulfur atoms. The corresponding kappa indexes for such chelate complexes
would be κ3O1,O7,O13 and κ3S4,S10,S16, respectively.
Examples 9, 10 and 11 illustrate three modes of chelation of the ligand N-[N-(2-aminoethyl)N',S-diphenylsulfonodiimidoyl]benzamidine. The use of kappa indexes allows these binding
modes (and others) to be distinguished and identified, in spite of the abundance of
heteroatoms that could coordinate.
Examples:
DRAFT 2 April 2004
Page 20 of 69
21
9.
HN
s
S
Ph
N
tio
n
C
+
NPh
H
N
Ph
CH2
Cu
CH2
da
N
H2
om
me
n
Cl
{N-[N-2-(amino-κN-ethyl)-N'-phenylbenzenesulfonodiimidoyl-κN]benzenimidamideκN'}chloridocopper(II)
10.
+
NH
Ph
C
Ph
ec
HN
lR
S
N
N
CH2
Cu
Cl
N
H2
CH2
isi
o
na
Ph
ov
{N-[N-2-(amino-κN-ethyl)-N'-phenylbenzenesulfonodiimidoylκ2N,N']benzenimidamide}chloridocopper(II)
IU
PA
C
Pr
11.
Page 21 of 69
PhN
+
Ph
S
HN
C
NH
Ph
N
CH2
Cu
Cl
N
H2
CH2
{N-[N-2-(amino-κN-ethyl)-N'-phenylbenzenesulfonodiimidoyl-κN]benzenimidamideκN'}chloridocopper(II)
DRAFT 2 April 2004
22
tio
n
s
The distinction between the names in Examples 9 and 11 rests on the conventional priming
of the second nitrogen atom in the benzenimidamide functional group. The prime identifies
the imino benzenimidamide nitrogen atom, which is more remote from the 2-aminoethyl
group (where the name began).
Examples:
12.
om
me
n
da
The use of donor atom locants on the atomic symbols to indicate point-of-ligation is
illustrated by the two isomeric bidentate modes of binding of the macrocycle 1,4,7triazacyclodecane (Examples 12 and 13). Conveying the formation of the five-membered
chelate ring requires the index κ2N1,N4, while the six-membered chelate ring requires the
index κ2N1,N7. The naming of a complex structure is shown in Example 14.
8
9
10
7
N
1
6
ec
N
lR
M
IU
PA
C
isi
o
Pr
ov
13.
na
4
2
5
N
3
κ2N1,N4
3
2
N
N
1
5
M
7
N
4
10
9
6
8
κ2N1,N7.
14.
DRAFT 2 April 2004
Page 22 of 69
23
NH2
N
6
2
4
9
8
CH2OH
4'
1'
2'
3'
9
N
N
1'
2'
4'
3'
OH
O
ec
lR
na
isi
o
ov
5')-2'-deoxycytidylyl(3'
κN7]platinum(II)
O
5'
O
P
CH2
O
O-
3'
4'
O
2'
1'
N
O
N
NH2
5')-2'-deoxyguanosinato(2_)-
Comparison of the eta and kappa nomenclatures
Pr
IR-9.2.4.3
P
O
5'
CH2
diammine[2'-deoxyguanylyl-κN7-(3'
O-
O
O
H 2N
5'
O
N
8
3
tio
n
7
7
5
HN 1
3
da
N
4
om
me
n
O
N
Pt
N
5
s
6
NH3
H 3N
2
1
O
PA
C
The modifiers eta, η, and kappa, κ, are complementary and should not be interchanged in
IU
usage. The eta nomenclature (Section IR-10.2.5.1) is used to specify the number of
contiguous donor atoms involved in the bond to a central atom (hapticity). It is required only
when there is more than one ligand atom involved in the bond to the central atom (i.e. the
term η1 is not required). The contiguous atoms are often the same element, but need not be.
Page 23 of 69
The kappa nomenclature is used to specify the point of attachment to the metal in the event a
ligand contains several different possible points of attachment (that are not all used), or to
DRAFT 2 April 2004
24
s
specify the point of attachment of the same ligand to different metal centres in a polynuclear
complex.
Donor atom symbol to indicate points of ligation
da
IR-9.2.4.4
tio
n
In cases where two or more identical ligands (or parts of a polydentate ligand) are bound to a
central atom, a superscript may be used on κ to indicate the number of donor atoms.
ec
om
me
n
Donor atoms of a ligand may be denoted by adding the italicized symbol(s) for the donor
atom (or atoms) to the end of the name (or formula) of the ligand. Thus, the 1,2-dithiooxalate
anion may conceivably be attached through S or O, and possible bidentate coordination
modes are distinguished by indicating the donor atoms: 1,2-dithiooxalato-S,S'; 1,2dithiooxalato-O,O'; 1,2-dithiooxalato-O,S; 1,2-dithiooxalato-O,S'. A similar approach may
be used for ligands such as thiocyanate and nitrite. The following system of citation of
ligating atoms is suggested for simple cases. (The kappa convention should be used for
complicated cases).
isi
o
Examples:
1.
na
lR
For ligands with ligating atoms linearly arranged along a chain, the order of citation symbols
should be successive, starting at one end. The choice of end is based upon alphabetical order
if the ligating atoms are different, e.g. cysteinato-N,S; cysteinato-N,O. When no such simple
distinction can be made, the ligating atom at the site with the lowest locant assigned according
to organic practice (see Section P-14.3.4 of Ref. 1) is given preference.
(CF3COCHCOMe)-
ov
1,1,1-trifluoro-2,4-dioxopentan-3-ido-O would be used to refer to
coordination of the CF3CO portion of the molecule, since it is preferred to
Pr
(i.e. has a lower locant than) MeCO, which would be identified by O'.
IU
PA
C
2.
H2NCH(Me)CH2NH2
propane-1,2-diamine-N would be used to refer to coordination of the
NH2CH2 portion of the molecule, since it is preferred to (i.e. has a lower
locant than) NH2CHMe, which would be identified by N'.
If the same element is involved in the different positions, the place in the chain or ring to
which the central atom is attached may alternatively be indicated by numerical superscripts. It
may be necessary to provide numerical superscripts where none is available from normal
organic substitutive nomenclature.
DRAFT 2 April 2004
Page 24 of 69
25
(MeCOCHCOMe)-
s
3.
Polynuclear complexes
IR-9.2.5.1
General
om
me
n
da
IR-9.2.5
tio
n
2,4-dioxopentan-3-ido-C3 indicates coordination through the central carbon
atom.
Polynuclear inorganic compounds exist in a bewildering array of structural types, such as
ionic solids, molecular polymers, extended assemblies of oxoanions, chains and rings,
bridged metal complexes, and homo- and hetero-nuclear clusters. This section primarily
treats the nomenclature of bridged metal complexes and homo- and hetero-nuclear clusters.
Coordination polymers are treated extensively elsewhere.7
na
lR
ec
As a general principle, as much structural information as possible should be presented when
writing the name or formula of a polynuclear complex. However, polynuclear complexes may
have structures so large and extended as to make a rational structure-based nomenclature
impractical. Furthermore, their structures may be undefined or not suitably elucidated. In
such cases, the principal function of the name or formula is to convey the stoichiometric
proportions of the various moieties present.
ov
isi
o
Ligands are cited in the usual alphabetical order with appropriate numerical prefixes. The
central atoms are listed using the atom priority derived from Table VI; the first reached on
following the direction of the arrow is placed last. The central atoms are placed after the
ligands when naming a complex and before the ligands in the formula of the complex.
PA
C
Pr
The number of central atoms of a given kind, if greater than one, is indicated in names by a
numerical prefix (Example 1). For anionic species, the suffix 'ate' and the number indicating
the charge on the ion are added after the central atom list; the list of central atoms is then
enclosed in parentheses (Example 2).
IU
Where necessary, the symbol kappa, κ, with the italicized atomic symbol(s) of the donor(s) is
employed to indicate the ligating atom(s) (Section IR-9.2.4.2). The distribution of ligands
between the central atoms is indicated by including a number, to the left of each kappa
symbol, which refers to the position of the central atom in the list at the end of the name. This
number is the priority of the central atom derived from Table VI (Example 3). Thus the
inclusion of (benzenethiolato-1κS) in a name would indicate that the sulfur atom of
benzenethiolate is bonded to central atom number 1.
Page 25 of 69
DRAFT 2 April 2004
26
[Rh3H3{P(OMe)3}6]
om
me
n
Examples:
1.
da
tio
n
s
These general rules may be relaxed in order to provide additional information, if available.
For example, the order of symbols in a formula may be changed to reflect the structure of a
molecule. In this case it is preferable to write the formula of the ligand so that the donor atom
is placed close to the central atom to which it is attached. More details are given in the
following sections.
trihydridohexakis(trimethyl phosphite)trirhodium
2.
S
PhSFe
FeSPh
2-
S
MoSPh
lR
S
S
ec
PhSMo
_
na
[Mo2Fe2S4(SPh)4]2
3.
isi
o
tetrakis(benzenethiolato)-1κS,2κS,3κS,4κS-tetra-µ3-sulfido-tetrahedro(dimolybdenumdiiron)ate(2_)
1 2
Bridging ligands
Pr
IR-9.2.5.2
ov
[(OC)5ReCo(CO)4]
nonacarbonyl-lκ5C,2κ4C-rheniumcobalt(Re—Co)
PA
C
Bridging ligands, as far as they can be specified, are indicated by the Greek letter µ appearing
before the ligand name and separated from it by a hyphen. The whole term, e.g. µ-chlorido, is
separated from the rest of the name by hyphens, as in ammine-µ-chlorido-chlorido, etc., or by
IU
parentheses if more complex ligands are involved. If the bridging ligand occurs more than
once and multiplicative prefixes are employed, the presentation is modified, as in tri-µchlorido-chlorido, etc., or as in bis(µ-diphenylphosphido), etc. if more complex ligands are
involved. The kappa nomenclature can be used when it is necessary to specify which central
atoms are bridged and through which donor atoms (see Example 4 of Section IR-9.2.5.5.)
DRAFT 2 April 2004
Page 26 of 69
27
s
The bridging index, the number of coordination centres connected by a bridging ligand, is
indicated by a right subscript, µn, where n > 2. The bridging index 2 is not normally indicated.
tio
n
Bridging ligands are listed in alphabetical order along with the other ligands, but a bridging
ligand is cited before a corresponding non-bridging ligand, as with di-µ-chloridotetrachlorido, unless structural information is being provided.
IR-9.2.5.3
om
me
n
da
Multiple bridging is listed in descending order of complexity, e.g. µ3-oxido-di-µ-oxidotrioxido. For ligand names requiring enclosing marks, µ is contained within those marks.
Metal-metal bonding
na
lR
ec
Metal-metal bonding may be indicated in names by placing italicized atomic symbols of the
appropriate metal atoms, separated by an 'em' dash and enclosed in parentheses, after the list
of central atoms and before the ionic charge. The symbols are placed in the same order as the
central atoms appear in the name (i.e. according to Table VI, with the first element reached
when following the arrow being placed last). The number of such metal-metal bonds is
indicated by an arabic numeral placed before the first element symbol and separated from it
by a space. For the purpose of nomenclature, no distinction is made between different metalmetal bond orders.
Examples:
1.
_
[Br4ReReBr4]2
2.
isi
o
bis(tetrabromidorhenate)(Re—Re)(2_)
[Mn2(CO)10]
3.
ov
bis(pentacarbonylmanganese)(Mn—Mn)
1 2
Pr
[(OC)5ReCo(CO)4]
nonacarbonyl-lκ5C,2κ4C-rheniumcobalt(Re—Co)
PA
C
4.
IU
IR-9.2.5.4
Page 27 of 69
Cs3[Re3Cl12]
caesium dodecachlorido-triangulo-trirhenate(3 Re—Re)(3_)
Symmetrical dinuclear entities
The order of ligands and central atoms may be modified if the symmetry of the entity permits
simpler names to be formed by employing multiplicative prefixes.
DRAFT 2 April 2004
28
s
[{PtCl(PPh3)}2(µ-Cl)2]
di-µ-chlorido-bis[chlorido(triphenylphosphane)platinum]
3.
[{Fe(NO)2}2(µ-PPh2)2]
bis(µ-diphenylphosphanido)bis(dinitrosyliron)
da
2.
om
me
n
IR-9.2.5.5
[{Cr(NH3)5}2(µ-OH)]5+
µ-hydroxido-bis(pentaamminechromium)(5+)
tio
n
Examples:
1.
Unsymmetrical dinuclear entities
The name of an unsymmetrical dinuclear species will result from following the general rules
described in Section IR-9.2.5.1. These will give an alphabetical list of ligands followed by a
priority list of central atoms (derived from the position of the central atoms in Table VI).
lR
ec
In Example 1 below, iridium is reached last on following the arrow shown in Table VI and is
therefore listed before mercury in the name. It is also given priority number 1.
isi
o
na
More detailed structural information is provided by including the kappa symbols for each
ligand. This allows the donor atoms to be specified using the donor atom symbol
immediately to the right of the kappa symbol. Placing the central atom priority number to the
left of the kappa symbol specifies the central atom to which ligands are attached.
Pr
ov
In cases where the central atoms are the same but have different coordination environments,
the priority number is assigned by identifying the first point in the name where the ligands
are not evenly distributed between the central atoms. Therefore, the central atom with the
greater number of ligands earlier in the name has priority. Thus, in Example 2 the chromium
atom with five of the nine ammine ligands attached is given priority number 1.
IU
PA
C
Bridging is indicated by the prefix µ and where bridging is accomplished by different atoms
of the same group, the ligating locants and symbols are separated by a colon, e.g. -µ-nitrito1κΝ:2κO-. In general, the hierarchy of punctuation marks is comma lower than colon lower
than semi-colon but in this context the colon is used only to indicate bridging. Thus, in
Example 5, both the comma and the semicolon appear since the bpy ligands are not involved
in bridging and the modified hierarchy of marks must be applied.
Examples:
1.
1
2
[{IrCl2(CO)(PPh3)2}(HgCl)]
DRAFT 2 April 2004
Page 28 of 69
29
2.
1
tio
n
s
carbonyl-1κC-trichlorido-1κ2Cl,2κCl-bis(triphenylphosphane1κP)iridiummercury(Ir—Hg)
2
[Cr(NH3)5(µ-OH)Cr(NH3)4(NH2Me)]5+
[{Co(NH3)3}2(µ-NO2)(µ-OH)2]3+
om
me
n
3.
da
nonaammine-1κ5N,2κ4N-µ-hydroxido-(methanamine-2κN)dichromium(5+)
di-µ-hydroxido-µ-nitrito-κN:κO-bis(triamminecobalt)(3+)
4.
1
2
[(H3N)3Co(µ-OH)2(µ-NO2)Co(py)(NH3)2]3+
5.
1
ec
pentaammine-1κ3N,2κ2N-di-µ-hydroxido-µ-nitrito-1κΝ:2κΟ-(pyridine2κN)dicobalt(3+)
2
lR
[(bpy)(H2O)Cu(µ-OH)2Cu(bpy)(SO4)]
6.
na
aqua-1κO-bis(2,2'-bipyridine)-1κ2N1,N1';2κ2N1,N1'-di-µ-hydroxido[sulfato(2_)-2κΟ]dicopper(II)
[{Cu(py)}2(µ-O2CMe)4]
Trinuclear and larger structures
ov
IR-9.2.5.6
isi
o
tetrakis(µ-acetato-κO:κO')bis[(pyridine)copper(II)]
IU
PA
C
Pr
The structural nomenclature of more complex polynuclear entities is based on the description
of the central or fundamental structural unit and a logical procedure for numbering the atoms.
Only the metal atoms are considered for this purpose. For nonlinear clusters, descriptors
such as tetrahedro and dodecahedro are used to describe a central structural unit (CSU).
However, synthetic chemistry has now advanced far beyond the range of the limited CSU set
associated with this usage (see below) and these descriptors should only be used for simple
cases.
A more comprehensive CSU descriptor and a numbering system, the CEP (Casey, Evans,
Powell) system, has been developed specifically for fully triangulated polyboron polyhedra
(deltahedra).8 The CEP descriptors are systematic alternatives to the traditional descriptors
for fully triangulated polyhedra (deltahedra) and are listed in Table IR-9.1.
Page 29 of 69
DRAFT 2 April 2004
30
triangulo
quadro
tetrahedro
D3h
D4h
Td
D3h
Oh
D3h
S6
D2d
Oh
Ih
octahedro
triprismo
antiprismo
dodecahedro
hexahedro (cube)
icosahedro
CEP descriptor
tio
n
Point group
[Td-(13)-∆4-closo]
[D3h-(131)-∆6-closo]
[Oh-(141)-∆8-closo]
da
3
4
4
5
6
6
8
8
8
12
Descriptor
om
me
n
Number of
atoms in CSU
s
Table IR-9.1 Structural descriptors
[D2d-(2222)-∆6-closo]
[Ih-(1551)-∆20-closo]
isi
o
na
lR
ec
Numbering of the CSU is based on locating a reference axis and planes of atoms
perpendicular to the reference axis. The reference axis is the axis of highest rotational
symmetry. Select that end of the reference axis with a single atom (or smallest number of
atoms) in the first plane to be numbered. Orient the CSU so that the first position to receive a
locant in the first plane with more than one atom is in the twelve o'clock position. Assign
locant numbers to the axial position or to each position in the first plane, beginning at the
twelve o'clock position and moving in either the clockwise or anticlockwise direction.
Pr
ov
From the first plane move to the next position and continue numbering in the same direction
(clockwise or anticlockwise), always returning to the twelve o'clock position or the position
nearest to it in the forward direction before assigning locants in that plane. Continue
numbering in this manner until all positions are numbered.
PA
C
A complete discussion of numbering deltahedra may be found elsewhere.8 The complete
descriptor for the CSU should appear just before the central atom list. Where structurally
significant, metal-metal bonds may be indicated (see Section IR-9.2.5.3).
IU
IR-9.2.5.7
Polynuclear clusters: symmetrical central structural units
Central structural units may be identified specifically and numbered for nomenclature as
described in Section IR-9.2.5.6. However, many symmetrical CSUs may not require a full
set of locants in the name because compounds based upon them do not exhibit isomerism.
DRAFT 2 April 2004
Page 30 of 69
31
tio
n
s
Locants for bridging ligands are cited as for dinuclear entities. Compound locants will at
times be necessary for monoatomic bridges in more complicated polynuclear entities. For
these entities the locants are cited before the ligand indicator κ and separated by a colon, e.g.
tri-µ-chlorido-1:2κ2Cl;1:3κ2Cl;2:3κ2Cl- indicates that there are three bridging chloride
2.
om
me
n
Examples:
1.
da
ligands and they bridge between atoms 1 and 2, 1 and 3, and 2 and 3 in the central atom list at
the end of the name. Note that because of the use of the colon, sets of bridge locants are
separated by semicolons. This practice applies the hierarchy of punctuation marks cited in
Section IR-9.2.5.5.
[{Co(CO)3}3(µ3-CBr)]
(µ3-bromomethanetriido)-nonacarbonyl-triangulo-tricobalt(3 Co—Co),
or (µ3-bromomethanetriido)triangulo-tris(tricarbonylcobalt)(3 Co—Co)
Cs3[Re3Cl12]
ec
caesium dodecachlorido-triangulo-trirhenate(3 Re—Re)(3_)
[Cu4(µ3-I)4(PEt3)4]
tetra-µ3-iodido-tetrakis(triethylphosphane)-tetrahedro-tetracopper, or
tetra-µ3-iodido-tetrakis(triethylphosphane)[Td-(13)-∆4-closo]tetracopper
4.
[Co4(CO)12]
ov
isi
o
na
lR
3.
Co 4
OC
OC
OC
Pr
PA
C
IU
CO
OC
1
CO
Co
Co
2
Co
CO
C
O
3
CO
CO
tri-µ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl1κ2C,2κ2C,3κ2C,4κ3C[Td-(13)-∆4-closo]tetracobalt(6 Co—Co)
The compound may also be named by using chain and ring nomenclature (see Section IR7.5 and Section II-5.3.3.3.6 of Ref. 9).
5.
Page 31 of 69
CO
OC
[Mo6S8]2
_
DRAFT 2 April 2004
6.
I
PtMe3
I
Me3Pt
I
I
om
me
n
PtMe3
da
Me3Pt
tio
n
octa-µ3-sulfido-octahedro-hexamolybdate (2_), or
octa-µ3-sulfido-[Oh-(141)-∆8-closo]hexamolybdate(2_)
s
32
tetra-µ3-iodido-tetrakis[trimethylplatinum(IV)], or
tetra-µ3-iodido-dodecamethyl-1κ3C,2κ3C,3κ3C,4κ3C-tetrahedro-tetraplatinum(IV), or
tetra-µ3-iodido-dodecamethyl-1κ3C,2κ3C,3κ3C,4κ3C-[Td-(13)-∆4-closo]tetraplatinum(IV)
[Be4(µ-O2CMe)6(µ4-O)]
ec
7.
[(HgMe)4(µ4-S)]2+
na
8.
lR
hexakis(µ-acetato-κΟ:κΟ')-µ4-oxido-tetrahedro-tetraberyllium, or
hexakis(µ-acetato-κΟ:κΟ')-µ4-oxido-[Td-(13)-∆4-closo]tetraberyllium
IU
PA
C
Pr
ov
9.
isi
o
µ4-sulfido-tetrakis(methylmercury)(2+), or
tetramethyl-1κC,2κC,3κC,4κC-µ4-sulfido-tetrahedro-tetramercury(2+), or
tetramethyl-1κC,2κC,3κC,4κC-µ4-sulfido[Td-(13)-∆4-closo]tetramercury(2+)
Ph3P
Pt
O
C
OC
Fe
PPh3
O
C
3
1
2
CO
Fe
CO
OC
C
O
C
O
octacarbonyl-1κ4C,2κ4C-bis(triphenylphosphane-3κP)-triangulodiironplatinum(Fe—Fe)(2 Fe—Pt)
DRAFT 2 April 2004
Page 32 of 69
33
IR-9.2.5.8
Polynuclear clusters: unsymmetrical central structural units
Beginning at each end of the chain, and working towards the centre, locate:
da
(i)
tio
n
s
Central atoms in chain, branched-chain, and cyclic polynuclear structures are numbered
consecutively from one end along the path containing the greatest number of central atoms.
For chains, the end from which numbering should begin is identified in the following way.
om
me
n
(a) the first points of central atom difference or, if the central atoms are symmetrically distributed
through the chain;
(b) the first central atoms at which there is a difference in the number of ligands
attached to a central atom or, if the ligands are symmetrically distributed through the
chain;
(c) the first central atoms where different types of ligand are attached.
ec
Compare the points of difference and begin numbering at the end of the chain closer
to:
lR
(ii)
na
(a) the central atom that is reached last on following the arrow shown in Table VI (i.e.
the central atom that appears first in the list of central atoms in the name, see Section
IR-9.2.5.1);
isi
o
(b) the central atom with more ligands;
ov
(c) the central atom with the ligand that comes first in the alphabetical order of
ligands.
Pr
When needed, the locant precedes the atom name in the central atom list, within the name (see
Example 1). The ligand indicator kappa, κ, (with central atom locant and italicized donor atom
symbol) is used where necessary to indicate the positions of the ligating atoms.
IU
PA
C
Examples:
1.
Page 33 of 69
DRAFT 2 April 2004
34
O
HO
H2
C
OH
O
2
H 3N
N
Co
1
O
Cr
NH3
H 3N
s
Co
O
OH2
da
H 3N
3+
NH3
3
tio
n
H3N
om
me
n
N
O
O
O
2.
[Os3(CO)12(SiCl3)2]
CO
Os
na
Cl3Si
lR
O
C
ec
hexaammine-2κ3N,3κ3N-aqua-1κO-{µ3-(1,2-ethanediyldinitrilo-1κ2N,N')-tetraacetato1κ3O1,O2,O3:2κO4:3κO4'}-di-µ-hydroxido-2:3κ4O-chromiumdicobalt(3+)
isi
o
OC
C
O
O
C
CO
Os
OC
C
O
O
C
CO
Os
OC
SiCl3
C
O
dodecacarbonyl-1κ4C,2κ4C,3κ4C-bis(trichlorosilyl)-1κSi,3κSi-
IU
PA
C
Pr
3.
ov
triosmium(2 Os—Os)
+
Ph
Ph2P
P
PPh2
CO
OC
1
2
Rh
Rh
Rh
OC
Cl
Ph2P
Cl
3
P
PPh2
Ph
DRAFT 2 April 2004
Page 34 of 69
35
tricarbonyl-1κC,2κC,3κC-µ-chlorido-1:2κ2Cl-chlorido-3κCl-bis{µ3-
tio
n
or, using the preferred IUPAC name1 for the phosphine ligand:
s
bis[(diphenylphosphanyl)-1κP':3κ P''-methyl]phenylphosphane-2κP}trirhodium(1+)
om
me
n
da
tricarbonyl-1κC,2κC,3κC-µ-chlorido-1:2κ2Cl-chlorido-3κCl-bis{µ3[phenylphosphanediyl-2κP-bis(methylene)]bis(diphenylphosphane)-1κP':3κP'' }trirhodium(1+)
Numbering cyclic structures requires location of a starting point and a direction for the
numbering. The central atom starting point is identified by comparing all the central atoms in
the cyclic portion of the molecule. The starting point will be:
ec
(i) the central atom that is reached last on following the arrow in Table VI (i.e. the central
atom that appears first in the list of central atoms in the name, see Section IR-9.2.5.1) and, if
there is more than one such central atom,
(ii) the atom with more ligands attached and, if the number of ligands is the same,
lR
(iii) the atom with the greatest number of alphabetically preferred ligands.
isi
o
na
If these highest ranked central atoms are still identical then the choice is made by comparing
the adjacent central atoms in the ring to one another, according to the criteria above, until a
difference is established.
ov
The direction of numbering from the starting point is determined by making this same
comparison of adjacent central atoms, but in this case comparing those that lie in each
direction from the chosen starting point.
Pr
The prefix 'cyclo', italicized and cited before all ligands, may be used for monocyclic
compounds.
IU
PA
C
Examples:
6.
Page 35 of 69
[Pt3(NH3)6(µ-OH)3]3+
cyclo-tri-µ-hydroxido-tris(diammineplatinum)(3+), or
hexaamminetri-µ-hydroxido-triangulo-triplatinum(3 +)
7.
DRAFT 2 April 2004
36
H3N
NH2Me
Pd
3
2
Pt 1
O
H
H 3N
s
NH3
Pt
NH3
tio
n
H3N
OH
da
HO
8.
N
(CO)2Rh
N
om
me
n
cyclo-pentaammine-1κ2N,2κ2N,3κN-tri-µ-hydroxido-1:2κ2O;1:3κ2O;2:3κ2O(methanamine-3κN)diplatinumpalladium(3+)
N
Rh (CO)2
N
ec
Me
lR
Me
N
Me
N
N
N
Rh (CO)2
isi
o
na
(CO)2Rh
Me
IU
PA
C
Pr
9.
ov
cyclo-tetrakis(µ-2-methylimidazolato-κN 1:κN3)tetrakis(dicarbonylrhodium)
N
(CO)2Rh
N
1
Rh (CO)(PMe3)
4
N
N
Me
Me
Me
Me
N
N
3
2
(Me3P)(CO) Rh
N
DRAFT 2 April 2004
N
Rh (CO)2
Page 36 of 69
37
cyclo-hexacarbonyl-1κ2C,2κC,3κ2C,4κC-tetrakis(µ-2-methylimidazolato)-
s
1:2κ2N1:N3;1:4κ2N1:N3;2:3κ2N1:N3;3:4κ2N1:N3-bis(trimethylphosphane)-2κP,4κP-tetrarhodium
da
[Os3(CO)12]
cyclo-dodecacarbonyl-1κ4C,2κ4C,3κ4C-triosmium(3 Os—Os)
om
me
n
Example:
10.
tio
n
When the prefix cyclo is used in the names of metal-metal bonded entities, symbols
indicating the presence of the metal-metal bonds are required.
IR-9.3
DESCRIBING THE CONFIGURATION OF COORDINATION COMPOUNDS
IR-9.3.1
Introduction
isi
o
na
lR
ec
Once the constitution of a compound has been defined, it remains to describe the spatial
relationships between the structural components of the molecule. Molecules that differ only
in the spatial distribution of the components are known as stereoisomers. Stereoisomers that
are mirror images of one another are called enantiomers (sometimes these have been called
optical isomers), while those that are not are called diastereoisomers (or geometrical isomers).
This is an important distinction in chemistry as, in general, diastereoisomers exhibit different
physical and spectroscopic properties from one another, while enantiomers exhibit identical
properties (except in the presence of other chiral entities). It is instructive to consider an
everyday analogy in order to establish how the configuration of a molecule (and the
embedded spatial relationships) can be described.
IU
PA
C
Pr
ov
Using the terminology introduced above, left and right hands may be regarded as enantiomers
of one another, since they are different (non-superimposable), but they are mirror images of
each other. In both cases the thumbs are adjacent to the index finger, and the components of
each hand are similarly disposed relative to all the other parts of that hand. If the thumb and
index finger of a right hand were to be exchanged, the resulting hand could be considered to
be a diastereoisomer of the normal right hand (and it too would have an enantiomer, resulting
from a similar exchange on a left hand). The key point is that the relative positions of the
components of diastereoisomers (the normal right hand and the modified one) are different.
Page 37 of 69
In order to describe the hand fully the components (four fingers, one thumb, and the central
part of the hand) must be identified, the points of attachment available on the hand, and the
relative positions of the fingers and thumb around the hand, must be described and whether
the hand is 'left' or 'right' must be identified. The last three steps deal with the stereochemistry
of the hand.
DRAFT 2 April 2004
38
tio
n
s
In the case of a coordination compound, the names and formula describe the ligands and
central atom(s). Describing the configuration of such a coordination compound requires
consideration of three factors:
(i) coordination geometry – identification of the overall shape of the molecule;
om
me
n
da
(ii) relative configuration – description of the relative positions of the components of the
molecule, i.e. where the ligands are placed around the central atom(s) in the identified
geometry;
(iii) absolute configuration – identification of which enantiomer is being specified (if the
mirror images are non-superimposable).
The next three sections deal with these steps in turn. A more detailed discussion of the
configuration of coordination compounds can be found elsewhere.10
Describing the coordination geometry
IR-9.3.2.1
Polyhedral symbol
lR
ec
IR-9.3.2
ov
isi
o
na
Different geometrical arrangements of the atoms attached to the central atom are possible for
all coordination numbers greater than one. Thus, two-coordinate species may involve a linear
or a bent disposition of the ligands and central atom. Similarly, three-coordinate species may
be trigonal planar or trigonal pyramidal, and four-coordinate species may be square planar,
square pyramidal, or tetrahedral. The coordination polyhedron (or polygon in planar
molecules) may be denoted in the name by an affix called the polyhedral symbol. This
descriptor distinguishes isomers differing in the geometries of their coordination polyhedra.
IU
PA
C
Pr
The polyhedral symbol indicates the geometrical arrangements of the coordinating atoms
about the central atom. This symbol must be assigned before any other stereochemical
features can be considered. It consists of one or more capital italic letters derived from
common geometric terms which denote the idealized geometry of the ligands around the
coordination centre, and an arabic numeral that is the coordination number of the central
atom.
Distortions from idealized geometries commonly occur. However, it is normal practice to
relate molecular structures to idealized models. The polyhedral symbol is used as an affix,
enclosed in parentheses and separated from the name by a hyphen. The polyhedral symbols
for the most common geometries for coordination numbers 2 to 9 are given in Table IR-9.2
and the corresponding structures and/or polyhedra are shown in Table IR-9.3.
DRAFT 2 April 2004
Page 38 of 69
39
Polyhedral
number
tio
n
2
2
3
3
3
4
4
4
4
5
5
6
6
7
7
7
8
8
8
8
8
8
8
9
9
Pr
ov
isi
o
na
lR
ec
linear
angular
trigonal plane
trigonal pyramid
T-shaped
tetrahedron
square plane
square pyramid
see-saw
trigonal bipyramid
square pyramid
octahedron
trigonal prism
pentagonal bipyramid
octahedron, face monocapped
trigonal prism, square face monocapped
cube
square antiprism
dodecahedron
hexagonal bipyramid
octahedron, trans-bicapped
trigonal prism, triangular face bicapped
trigonal prism, square face bicapped
trigonal prism, square face tricapped
heptagonal bipyramid
symbol
L-2
A-2
TP-3
TPY-3
TS-3
T-4
SP-4
SPY-4
SS-4
TBPY-5
SPY-5
OC-6
TPR-6
PBPY-7
OCF-7
TPRS-7
CU-8
SAPR-8
DD-8
HBPY-8
OCT-8
TPRT-8
TPRS-8
TPRS-9
HBPY-9
da
Coordination
polyhedron
om
me
n
Coordination
s
Table IR-9.2 Polyhedral symbolsa
PA
C
a Strictly, not all geometries can be represented by polyhedra.
IU
Table IR-9.3 Polyhedral symbols, geometrical structures and/or polyhedra
Page 39 of 69
Three-coordination
trigonal plane
trigonal pyramid
DRAFT 2 April 2004
T-shape
TPY-3
TS-3
da
TP-3
tio
n
s
40
Four-coordination
square plane
om
me
n
tetrahedron
ec
T-4
see-saw
isi
o
na
lR
square pyramid
SP-4
ov
SPY-4
SS-4
Five-coordination
square pyramid
IU
PA
C
Pr
trigonal bipyramid
TBPY-5
SPY-5
Six-coordination
DRAFT 2 April 2004
Page 40 of 69
41
trigonal prism
da
tio
n
s
octahedron
TPR-6
om
me
n
OC-6
Seven-coordination
octahedron, face
monocapped
trigonal prism,
square face
monocapped
square
antiprism
TPRS-7
dodecahedron
hexagonal
bipyramid
PA
C
Pr
ov
cube
OCF-7
isi
o
Eight-coordination
na
PBPY-7
lR
ec
pentagonal
bipyramid
IU
CU-8
Page 41 of 69
octahedron,
trans-bicapped
SAPR-8
trigonal prism,
triangular face
bicapped
DRAFT 2 April 2004
DD-8
HBPY-8
trigonal prism,
square face
bicapped
TPRT-8
Nine-coordination
heptagonal
bipyramid
na
lR
ec
trigonal prism,
square face
tricapped
TPRS-8
om
me
n
OCT-8
da
tio
n
s
42
IR-9.3.2.2
HBPY-9
isi
o
TPRS-9
Choosing between closely related geometries
IU
PA
C
Pr
ov
For real molecules, the stereochemical descriptor should be based on the nearest idealized
geometry. However, some idealized geometries are closely related (e.g. square planar – SP-4,
four-coordinate square pyramidal – SPY-4, see-saw – SS-4, and tetrahedral – T-4; T-shaped –
TS-3, trigonal planar – TP-3, and trigonal pyramidal – TPY-3) and care may therefore be
required in making the choice.
The following approach is useful in determining the polyhedral symbol for four-coordinate
structures. The key is to consider the locations of the central atom and the coordinating
atoms in relation to each other. If all five atoms are in (or are close to being in) the same
plane, then the molecule should be treated as square planar. If the four coordinating atoms
are in a plane, but the central atom is significantly displaced from the plane, then the square
pyramidal geometry is appropriate. If the four coordinating atoms do not lie in (or close to) a
plane, then a polyhedron can be defined by joining all four coordinating atoms together with
DRAFT 2 April 2004
Page 42 of 69
43
s
lines. If the central atom lies inside this polyhedron the molecule should be regarded as
tetrahedral, otherwise, it should be regarded as having a see-saw structure.
da
tio
n
T-shaped and trigonal planar molecules both have a central atom that lies in (or close to) the
plane defined by the coordinating atoms. They differ in that the angles between the three
coordinating atoms are approximately the same in the trigonal planar structure, while one
angle is much larger than the other two in a T-shaped molecule. The central atom lies
significantly out of the plane in a trigonal pyramidal structure.
Describing relative configuration – distinguishing between diastereoisomers
IR-9.3.3.1
General
om
me
n
IR-9.3.3
ec
The placement of ligands around the central atom must be described in order to identify a
particular diastereoisomer. There are a number of common terms (e.g. cis, trans, mer and
fac) used to describe the relative locations of ligands in simple systems. However, they can be
used only when a particular geometry is present (e.g. octahedral or square planar), and when
there are only two kinds of donor atom present (e.g. Ma2b2 in a square planar complex,
lR
where M is a central atom and 'a' and 'b' are types of donor atom).
na
Several methods have been used to distinguish between diastereoisomers in more complex
systems. Thus, stereoisomers resulting from the coordination of linear tetradentate ligands
have often been identified as trans, cis-α, or cis-β,11 and those resulting from coordination of
ov
isi
o
macrocyclic tetradentate ligands have their own system.12 The scope of most of these
nomenclatures is generally quite limited, but a proposal with wider application in the
description of complexes of polydentate ligands has been made recently.13
PA
C
Pr
Clearly a general method is required in order to distinguish between diastereoisomers of
compounds in which either other geometries or more than two kinds of donor atoms are
present. The configuration index has been developed for this purpose. The next section
outlines the method by which a configuration index is obtained for a compound, and the
following sections give details for particular geometries. Commonly used terms are included
when the appropriate geometry is being discussed.
IU
IR-9.3.3.2
Page 43 of 69
Configuration index
Once the coordination geometry has been specified by the polyhedral symbol, it becomes
necessary to identify which ligands (or donor atoms) occupy particular coordination
positions. This is achieved through the use of the configuration index which is a series of
digits identifying the positions of the ligating atoms on the vertices of the coordination
DRAFT 2 April 2004
44
tio
n
s
polyhedron. The individual configuration index has the property that it distinguishes between
diastereoisomers. It appears within the parentheses enclosing the polyhedral symbol (see
Section IR-9.3.2.1), following that symbol and separated from it by a hyphen.
Each donor atom must be assigned a priority number based on the rules developed by Cahn,
Ingold and Prelog (the CIP rules).14 These priority numbers are then used to form the
om
me
n
da
configuration index for the compound. The application of the CIP rules to coordination
compounds is discussed in detail in Section IR-9.3.5 but, in general, donor atoms that have a
higher atomic number have higher priority than those that have a lower atomic number.
Square planar coordination systems (SP-4)
lR
IR-9.3.3.3
ec
The presence of polydentate ligands may require the use of primes on some of the numbers
in the configuration index. The primes are used to indicate either that donor atoms are not
part of the same polydentate ligand as those that have unprimed priority numbers, or that the
donor atoms belong to different parts of a polydentate ligand that are related by symmetry. A
primed priority number means that that donor atom has lower priority than the same kind of
donor atom without a prime on the priority number. More detail on the 'priming convention'
can be found in Section IR-9.3.5.3.
na
The terms cis and trans are used commonly as prefixes to distinguish between stereoisomers
in square planar systems of the form [Ma2b2], where M is the central atom, and 'a' and 'b' are
isi
o
different types of donor atom. Similar donor atoms occupy coordination sites adjacent to one
another in the cis isomer, and opposite to one another in the trans isomer. The cis-trans
terminology is not adequate to distinguish between the three isomers of a square planar
coordination entity [Mabcd], but could be used, in principle, for an [Ma2bc] system (where
Pr
ov
the terms cis and trans would refer to the relative locations of the similar donor atoms). This
latter use is not recommended.
IU
PA
C
The configuration index for a square planar system is placed after the polyhedral symbol
(SP-4). It is the single digit which is the priority number for the ligating atom trans to the
ligating atom of priority number 1, i.e. the priority number of the ligand trans to the most
preferred ligating atom.
Examples:
1.
Priority sequence: a > b > c > d
Priority number sequence: 1 < 2 < 3 < 4
DRAFT 2 April 2004
Page 44 of 69
45
a
b
M
a
c
tio
n
d
da
c
s
SP-4-4
M
SP-4-2
b
a
om
me
n
d
b
M
SP-4-3
c
d
ec
2.
1
Pt
3
N
NCMe
1
Cl
isi
o
na
lR
Cl
2
(SP-4-1)-(acetonitrile)dichlorido(pyridine)platinum(II)
PA
C
Pr
ov
If there are two possibilities, as in Example 3, the configuration index is the priority number
with the higher numerical value. Both the priority 2 ligand (acetonitrile) and the priority 3
ligand (pyridine) are trans to a priority 1 ligand (chloride). The higher numerical value (3) is
chosen for the configuration index. This choice is sometimes referred to as having been
made according to the principle of trans maximum difference, i.e. that the difference between
the numerical values of the priority numbers of the ligands should be as large as possible.
IU
3.
Page 45 of 69
1
Cl
2
NCMe
Pt 3
Cl
N
1
DRAFT 2 April 2004
46
Octahedral coordination systems (OC-6)
tio
n
IR-9.3.3.4
s
(SP-4-3)-(acetonitrile)dichlorido(pyridine)platinum(II)
The terms cis and trans are used commonly as prefixes to distinguish between stereoisomers
in octahedral systems of the form [Ma4b2], where M is the central atom, and 'a' and 'b' are
om
me
n
da
different types of donor atom. The 'b' donors occupy adjacent coordination sites in the cis
isomer, and opposite coordination sites in the trans isomer (Example 1).
The terms mer (meridional) and fac (facial) are used commonly to distinguish between
stereoisomers of complexes of the form [Ma3b3]. In the mer isomer (Example 2) the two
ec
groups of three similar donors each lie on a meridian of the coordination octahedron, in
planes that also contain the central atom. In the fac isomer (Example 3) the two groups of
three similar donors each occupy coordination sites on the corners of a face of the
coordination octahedron.
na
lR
The configuration index of an octahedral system follows the polyhedral symbol (OC-6) and
consists of two digits. The first digit is the priority number of the ligating atom trans to the
ligating atom of priority number 1, i.e. the priority number of the ligand trans to the most
preferred ligating atom.
isi
o
If there is more than one ligand of priority 1, then the first digit is the priority number of the
trans ligand with the highest numerical value (remembering that a primed number will be of
higher numerical value than the corresponding unprimed number).
Pr
ov
These two ligating atoms, the priority 1 ligand and the (lowest priority) ligand trans to it,
define the reference axis of the octahedron.
IU
PA
C
The second digit of the configuration index is the priority number of the ligating atom trans
to the most preferred ligand in the plane that is perpendicular to the reference axis. If there is
more than one of the highest priority ligand in the plane, the priority number of the trans
ligand having the largest numerical value is selected.
Examples:
1.
DRAFT 2 April 2004
Page 46 of 69
47
1
a
2
2
b
b
2
b
2
a
2
om
me
n
b
2
a
ec
2
2.
lR
1
1
NO2
na
O2N
2
Co
1
NO2
isi
o
NH3
2
mer-[Co(NH3)3(NO2)3] or
(OC-6-21)-triamminetrinitrito-κ3N-cobalt(III)
Pr
ov
2
NH3
H 3N
PA
C
3.
IU
1
2
a
b
1
2
NO2
H 3N
2
1
NO2
Co
H 3N
1
NO2
NH3
2
fac-[Co(NH3)3(NO2)3] or
Page 47 of 69
OC-6-12
da
1
b
b
tio
n
s
b
DRAFT 2 April 2004
1
OC-6-22
48
(OC-6-22)-triamminetrinitrito-κ3N-cobalt(III)
AsPh3
4
CO
Cr
NCMe
3
C
O
ON
MeCN
om
me
n
3
da
2
tio
n
+
1
s
4.
4
(OC-6-43)-bis(acetonitrile)dicarbonylnitrosyl(triphenylarsane)chromium(1+)
IR-9.3.3.5
Square pyramidal coordination systems (SPY-4, SPY-5)
ec
The configuration index of an SPY-5 system consists of two digits. The first digit is the
priority number of the ligating atom on the C4 symmetry axis (the reference axis) of the
lR
idealized pyramid. The second digit is the priority number of the ligating atom trans to the
ligating atom with the lowest priority number in the plane perpendicular to the C4 symmetry
isi
o
na
axis. If there is more than one of the highest priority (most preferred) ligand in the
perpendicular plane, then the second digit is chosen to have the highest numerical value (i.e.
the less preferred ligand that is trans to a most preferred ligand).
Pr
ov
The configuration index of an SPY-4 system is a single digit that is chosen in the same way
as the second digit of SPY-5 systems. The configuration index of a four-coordinate square
pyramidal system will therefore be the same as that for the square planar structure that would
result from the ligands and the central atom being coplanar. The difference between the
structures is described by the polyhedral symbol rather than by the configuration index.
IU
PA
C
Examples:
1.
4
3
1
SPY-5-43
3
2
2.
DRAFT 2 April 2004
Page 48 of 69
49
1
Br
2
But2PhP
PPhBut2
s
2
tio
n
Pd
PPhBut2
Br
1
da
2
IR-9.3.3.6
om
me
n
(SPY-5-12)-dibromidotris[di-tert-butyl(phenyl)phosphane]palladium
Bipyramidal coordination systems (TBPY-5, PBPY-7, HBPY-8, and HBPY-9)
ec
The configuration index for bipyramidal coordination systems follows the appropriate
polyhedral symbol, and consists of two segments separated by a hyphen. The first segment
has two digits which are the priority numbers of the ligating atoms on the highest order
rotational symmetry axis, the reference axis. The lower number is cited first.
Trigonal bipyramid (TBPY-5)
IU
PA
C
Pr
Examples:
1.
ov
isi
o
na
lR
The second segment consists of the priority numbers of the ligating atoms in the plane
perpendicular to the reference axis. (For the trigonal bipyramid, this segment of the
configuration index is not required and is therefore omitted). The first digit is the priority
number for the preferred ligating atom, i.e. the lowest priority number in the plane. The
remaining priority numbers are cited in sequential order proceeding around the projection of
the structure either clockwise or anticlockwise, in whichever direction gives the lower
numerical sequence. The lowest numerical sequence is that having the lower number at the
first point of difference when the numbers are compared digit by digit from one end to the
other.
1
PPh3
OC
2
CO
2
3
Fe
4
2
CO
PPh3
5
TBPY-5-25
Page 49 of 69
1
2
1
(TBPY-5-11)-tricarbonylbis(triphenylphosphane)iron
DRAFT 2 April 2004
50
Pentagonal bipyramid (PBPY-7)
s
2.
tio
n
3
2
1
da
PBPY-7-34-12342 (not 12432)
3
om
me
n
2
4
4
T-shaped systems (TS-3)
ec
IR-9.3.3.7
See-saw systems (SS-4)
na
IR-9.3.3.8
lR
The configuration index for T-shaped systems follows the polyhedral symbol and consists
of a single digit, the priority number of the ligand on the stem of the T (as opposed to the
cross piece of the T).
1
ov
1
isi
o
The configuration index for see-saw systems consists of two digits, the priority numbers of
the two ligands separated by the largest angle. The number of lower numerical value is cited
first.
3
Pr
2
IU
PA
C
M
largest angle
largest angle
M
1
3
2
1
SS-4-11
SS-4-12
DRAFT 2 April 2004
Page 50 of 69
51
Describing absolute configuration – distinguishing between enantiomers
IR-9.3.4.1
General
tio
n
s
IR-9.3.4
om
me
n
da
There are two well-established, but fundamentally different, systems for distinguishing
between two enantiomers (stereoisomers that are mirror images of one another). The first,
based on the chemical constitution of the compound, involves the R/S convention used for
describing tetrahedral centres and the closely related C/A convention used for other
polyhedra. The R/S and C/A conventions use the priority sequence referred to in Section IR9.3.3.2, and detailed in Section IR-9.3.5, where the ligands are assigned a priority number
based (usually) on the atomic number of the donor atoms and their substituents.
The second is based on the geometry of the molecule, makes use of the skew-line convention,
and applies only to octahedral complexes. The two enantiomers are identified by the symbols
∆ and Λ in this system. The C/A nomenclature is not required for those chelate complexes
The R/S convention for tetrahedral centres
lR
IR-9.3.4.2
ec
where the skew-line convention is completely unambiguous (see Sections IR-9.3.4.11 9.3.4.14).
isi
o
na
The convention used to describe the absolute configurations of tetrahedral centres was
originally developed for carbon atom centres (see Ref. 14 and Section P-91 of Ref. 1) but
can be used for any tetrahedral centre. There is no need to alter the rules in treating
tetrahedral metal complexes.
Pr
ov
The symbol R is assigned if the cyclic sequence of priority numbers, proceeding from
highest priority, is clockwise when the viewer is looking down the vector from the tetrahedral
centre to the least preferred substituent (the substituent having the priority number with the
highest numerical value, i.e. 4), as in Example 1. An anticlockwise cyclic sequence is
assigned the symbol S (Example 2).
IU
PA
C
Examples:
1.
2.
1
1
M
M
3
2
R
Page 51 of 69
DRAFT 2 April 2004
2
3
S
52
tio
n
s
This system is most often used in conjunction with ligand configuration but can be applied
equally to tetrahedral metal centres. It has also been useful for pseudotetrahedral
organometallic complexes when, for example, cyclopentadienyl ligands are treated as if they
were monodentate ligands of high priority.
da
Example:
3.
om
me
n
1
4
Fe
I
2
CO
PPh3
ec
3
The R/S convention for trigonal pyramidal centres
na
IR-9.3.4.3
lR
T-4-S
ov
isi
o
Molecules containing a trigonal pyramidal centre (TPY-3) may exist as a pair of
stereoisomers. The configuration of this centre can be described in a similar way to that of a
tetrahedral centre. This is achieved through notional placement of a 'phantom atom' of low
priority in the coordination site that would create a tetrahedral centre from a trigonal
pyramidal centre. The centre can then be identified as R or S by the methods described above.
PA
C
Pr
The use of some bonding theories leads to the placement of a lone pair on a trigonal
pyramidal centre. If this is done, the absolute configuration of the centre is also described by
the R/S convention, in this case by placing the 'phantom atom' in the site that is occupied by
the lone pair. Examples of this practice may be found in the description of absolute
configurations for sulfoxides in which the alkyl substituents are different.
IU
IR-9.3.4.4
The C/A convention for other polyhedral centres
The R/S convention makes use of priority numbers for the determination of chirality at
tetrahedral centres, as detailed above. The same principles are readily extendable to
geometries other than tetrahedral.15 However, in order to avoid confusion, and to emphasize
the unique aspects of the priority sequence systems as applied to coordination polyhedra, the
symbols R and S are replaced by the symbols C and A when applied to other polyhedra.
DRAFT 2 April 2004
Page 52 of 69
53
da
tio
n
s
The procedure for arriving at ligating atom priorities is detailed in Section IR-9.3.5. Once the
ligand priorities have been assigned, the reference axis (and direction) appropriate to the
geometry is identified. The priority numbers of the ligands coordinated in the plane
perpendicular to the reference axis are then considered, viewing from the axial ligand of
higher priority.
IR-9.3.4.5
om
me
n
Beginning with the highest priority ligand in the plane perpendicular to the reference axis, the
clockwise and anticlockwise sequences of priority numbers are compared, and that with the
lower number at the first point of difference is chosen. If the chosen sequence results from a
clockwise reading of the priority numbers, then the structure is given the chirality symbol C,
otherwise it is given the symbol A.
The C/A convention for trigonal bipyramidal centres
lR
ec
The procedure is similar to that used for tetrahedral systems in the R/S convention, but it is
modified because of the presence of a unique reference axis (running through the two axial
donor atoms and the central atom).
IU
PA
C
Pr
Examples:
ov
isi
o
na
The structure is oriented so that the viewer looks down the reference axis, with the more
preferred ligand (having a priority number with lower numerical value) closer to the viewer.
Accordingly, the axial ligand with the lower priority lies beyond the central atom. Using this
orientation, the priority sequence of the three ligating atoms in the trigonal plane is examined.
If the sequence proceeds from the highest priority to the lowest priority in a clockwise
fashion, the chirality symbol C is assigned. Conversely, if the sequence from highest to
lowest priority (from lowest numerical index to highest numerical index) is anticlockwise, the
symbol A is assigned.
1
1
2
4
M
3
5
4
2
1. Chirality symbol = C
Page 53 of 69
M
5
DRAFT 2 April 2004
3
2. Chirality symbol = A
54
IR-9.3.4.6
The C/A convention for square pyramidal centres
tio
n
s
A procedure similar to that described in Section IR-9.3.4.4 is used for square pyramidal
structures. In the case of SPY-5 systems, the polyhedron is oriented so that the viewer looks
along the formal C4 axis, from the axial ligand toward the central atom. The priority numbers
om
me
n
da
of the ligands in the perpendicular plane are then considered, beginning with the highest
priority ligand (the one having the priority number of lowest numerical value). The clockwise
and anticlockwise sequences of priority numbers are compared, and the structure is assigned
the symbol C or A according to whether the clockwise (C) or anticlockwise (A) sequence is
lower at the first point of difference.
The chirality of an SPY-4 system is defined in a similar way. In this case, the viewer looks
along the formal C4 axis in such a way that the ligands are further away than the central
ec
atom. The priority numbers are then used to assign the symbol C or A, as for the SPY-5
system.
Examples:
lR
1
3
4
na
M
2
isi
o
5
1. Chirality symbol = C
5
2
M
3
4
2. Chirality symbol = A
The C/A convention for see-saw centres
ov
IR-9.3.4.7
1
IU
PA
C
Pr
The absolute configurations of see-saw complexes can be described using the C/A system.
The configuration index for see-saw systems consists of two digits, the priority numbers of
the two ligands separated by the largest angle. The higher priority ligand of these two is
identified and used as a point from which to view the two ligands not involved in the
configuration index. If moving from the higher priority ligand to the lower (through the
smaller angle) entails making a clockwise motion, the absolute configuration is assigned C.
An anti-clockwise direction results in the absolute configuration A.
DRAFT 2 April 2004
Page 54 of 69
55
s
1
tio
n
3
anti-clockwise looking from the top
M
da
1
om
me
n
2
SS-4-12-A
IR-9.3.4.8
The C/A convention for octahedral centres
lR
ec
The absolute configurations of some octahedral complexes can be described using either the
skew-line reference system (Section IR-9.3.4.11) or the C/A system. The first is used more
commonly, but the C/A system is more general and may be used for most complexes. The
skew-line reference system is only applicable to tris(bidentate), bis(bidentate) and closely
related systems.
Pr
Examples:
ov
isi
o
na
The reference axis for an octahedral centre is that axis containing the ligating atom of CIP
priority 1 and the trans ligating atom of lowest possible priority (highest numerical value)
(see Section IR-9.3.3.4). The atoms in the coordination plane perpendicular to the reference
axis are viewed from the ligand having that highest priority (CIP priority 1) and the
clockwise and anticlockwise sequences of priority numbers are compared. The structure is
assigned the symbol C or A, according to whether the clockwise (C) or anticlockwise (A)
sequence is lower at the first point of difference.
1
PA
C
4
IU
5
M
3
Page 55 of 69
1
1
3
6
M
6
2
1. Chirality symbol = C
4
5
4
M
5
2
2. Chirality symbol = A
DRAFT 2 April 2004
3
6
2
3. Chirality symbol = C
56
s
Example 4 shows the compound [CoBr2(en)(NH3)2]+ which has the polyhedral symbol
OC-6 and the configuration index 32. The chirality symbol is C.
2
NH3
3
M
Co
N
H2
da
H 2C
1
+
Br
Br
NH3
2
om
me
n
H 2C
H2
N
tio
n
Example:
4.
1
3
Example 5 shows the complex [RuCl(CO)H(PMe2Ph)3] which has the descriptor OC-6-24A. The chloride ligand has priority 1.
ec
Example:
5.
1
lR
Cl
3
PMe2Ph
OC
na
Ru
PMe2Ph
PMe2Ph
isi
o
H
2
M
4
2
2
Pr
ov
The assignment for polydentate ligands is illustrated by Example 6 which uses the priming
convention developed in Section IR-9.3.5. Note that priority number 2 is higher than 2'.
IU
PA
C
Example:
6.
DRAFT 2 April 2004
Page 56 of 69
57
1
tio
n
s
1'
2
Chirality symbol = A
2'
om
me
n
3
3'
IR-9.3.4.9
da
M
The C/A convention for trigonal prismatic centres
lR
ec
For the trigonal prismatic system, the configuration index is derived from the CIP priority
numbers of the ligating atoms opposite the triangular face containing the greater number of
ligating atoms of highest CIP priority. The chirality symbol is assigned by viewing the
trigonal prism from above the preferred triangular face and noting the direction of
progression of the priority sequence for the less preferred triangular face.
1
na
Examples:
2
isi
o
3
Pr
ov
5
3
6
4
4
1. Chirality symbol = C
IU
Page 57 of 69
6
5
2. Chirality symbol = A
The C/A convention for other bipyramidal centres
PA
C
IR-9.3.4.10
1
2
The procedure used for the trigonal bipyramid is appropriate for other bipyramidal
structures. The structure is assigned the symbol C or A, according to whether the clockwise
(C) or anticlockwise (A) sequence is lower at the first point of difference when the numbers
are compared digit by digit from one end to the other (see Sections IR-9.3.4.5 and IR9.3.4.6) and the molecule is viewed from the higher priority ligand on the reference axis.
Example 1 has the descriptor PBPY-7-12-11'1'33-A.
DRAFT 2 April 2004
58
tio
n
s
Example:
1.
Me2N
1
C
S
Mo
NH3
S
Me2N
3
1'
OH2
3
1'
2
ec
The skew-lines convention
om
me
n
S
IR-9.3.4.11
da
NH3
S
C
1
lR
Tris(bidentate) complexes constitute a general family for which a useful unambiguous
convention has been developed based on the orientation of skew lines which define a helix.
na
Examples 1 and 2 represent the delta (∆) and lambda (Λ) forms of a complex such as
[Co(NH2CH2CH2NH2)3]3+. The rules define the chiralities of two additional families of
IU
PA
C
ov
Pr
Examples:
isi
o
structures. These are the cis-bis(bidentate) octahedral structures and the conformations of
certain chelate rings. It is possible to use the system described below for complexes of
higher polydentate ligands, but additional rules are required.16
1. delta (∆)
2. lambda (Λ)
Two skew-lines which are not orthogonal possess the property of having one, and only one,
normal in common. They define a helical system, as illustrated in Figures IR-9.1 and IR-9.2
(below). In Figure IR-9.1, one of the skew-lines, AA, determines the axis of a helix upon a
cylinder whose radius is equal to the length of the common normal, NN, to the two skewlines, AA and BB. The other of the skew-lines, BB, is a tangent to the helix at N and
DRAFT 2 April 2004
Page 58 of 69
59
A
da
tio
n
A
s
determines the pitch of the helix. In Figure IR-9.2, the two skew-lines AA and BB are seen in
projection onto a plane orthogonal to their common normal.
B
om
me
n
B
N
N
N
lR
ec
N
B
A
na
A
B
(b) Λ or λ
isi
o
(a) ∆ or δ
Figure IR-9.1. Two skew lines AA and BB which are not orthogonal define a helical system.
ov
In the Figure, AA is taken as the axis of a cylinder whose radius is determined by the common
normal NN of the two skew-lines. The line BB is a tangent to the above cylinder at its crossing
Pr
point with NN and defines a helix upon this cylinder. (a) and (b) illustrate a right- and lefthanded helix respectively.
B
IU
PA
C
B
A
A
B
(a) ∆ or δ
A
A
B
(b) Λ or λ
Figure IR-9.2. The figure shows pairs of non-orthogonal skew-lines in projection upon a
plane parallel to both lines. The full line BB is above the plane of the paper, the dotted line AA
Page 59 of 69
DRAFT 2 April 2004
60
is below this plane. Case (a) corresponds to (a) of Figure IR-9.1 and defines a right-handed
s
helix. Case (b) corresponds to (b) of Figure IR-9.1 and defines a left-handed helix.
da
tio
n
Parts (a) of Figures IR-9.1 and IR-9.2 illustrate a right-handed helix to be associated with the
Greek letter delta (∆ referring to configuration, δ to conformation). Parts (b) of Figures IR9.1 and IR-9.2 illustrate a left-handed helix to be associated with the Greek letter lambda (Λ
for configuration, λ for conformation). In view of the symmetry of the representation
IR-9.3.4.12
om
me
n
constituted by two skew-lines, the helix which the first line, say BB, determines around the
second, AA, has the same chirality as that which AA determines around BB. As one of the
lines is rotated about NN with respect to the other, inversion occurs when the lines are
parallel or perpendicular (Figure IR-9.1).
Application of the skew-lines convention to tris(bidentate) octahedral complexes
lR
ec
Any two of the three chelate rings may be chosen to designate the configuration of
tris(bidentate) coordination compounds. The donor atoms of each chelate ring define a line.
Two such lines for a pair of chelate rings in the same complex define a helix, one line being
the axis of the helix and the other a tangent of the helix at the normal common to the skewlines. The tangent describes a right-handed (∆) or a left-handed (Λ) helix with respect to the
axis and thereby defines the chirality of that configuration.
Application of the skew-lines convention to bis(bidentate) octahedral complexes
na
IR-9.3.4.13
ov
isi
o
Figure IR-9.3(a) shows a common orientation of an octahedral tris(bidentate) structure
projected onto a plane orthogonal to the three-fold axis of the structure. Figure IR-9.3(b)
shows the same structure oriented to emphasize the skew-line relationship between a pair of
chelate rings that can be used to define chirality. Figure IR-9.3(c) shows that the same
convention can be used for the cis-bis(bidentate) complex cis-[M(aa)2b2]. The two chelate
IU
PA
C
Pr
rings define the two skew-lines that, in turn, define the helix and the chirality of the
substance. The procedure is precisely the same as that described for the tris(bidentate) case,
but only a single pair of chelate rings is available.
(a)
(b)
(c)
Figure IR-9.3. Two orientations of a tris(bidentate) structure, (a) and (b), to show the chiral
relationship between these two species and bis(bidentate) structure (c).
DRAFT 2 April 2004
Page 60 of 69
61
IR-9.3.4.14
Application of the skew-lines convention to conformations of chelate rings
tio
n
s
In order to assign the chirality of a ring conformation, the line AA in Figure IR-9.2 is defined
as that line joining the two ligating atoms of the chelate ring. The other line BB is that joining
the two ring atoms which are neighbours to each of the ligating atoms. These two skew-lines
define a helix in the usual way. The tangent describes a right-handed (δ) or a left-handed (λ)
CH2
N
ec
N
om
me
n
da
helix with respect to the axis and thereby defines the conformation in terms of the convention
given in Figure IR-9.1. Non-helical situations may also give rise to chirality when the
chemical identities of the atoms are considered. For example, the chair and boat
conformations of six-membered chelate rings are not chiral. However, if the two donor atoms
are not identical then the chelate ring is chiral. Clearly the principles expounded in Section
IR-9.3.4.11 do not apply to such cases. The relationship between the convention of Figure
IR-9.2 and the usual representation of chelate ring conformation may be seen by comparing
Figures IR-9.2 and IR-9.4.
CH2
M
N
lR
M
CH2
CH2
CH2
(b)
na
(a)
N
isi
o
Figure IR-9.4. δ-Conformation chelate rings: (a) five-membered; (b) six-membered.
Determining ligand priority
IR-9.3.5.1
General
ov
IR-9.3.5
IU
PA
C
Pr
The methods for differentiating between stereoisomers outlined earlier in this chapter require
the assignment of priorities for the ligands attached to the central atom. These priority
numbers are then used in the configuration index, which describes the relative positions of
the ligands, and in the assignment of the absolute configuration of the compound.
Page 61 of 69
The following sections outline the method used to arrive at the priority numbers for a given
set of ligands (or donor atoms), and the ways that the basic rules have to be modified in order
to describe adequately systems that include polydentate ligands. These modifications, which
are collectively referred to as the priming convention, make use of primes on the priority
numbers to indicate which donor atoms atoms are grouped together within a particular
polydentate ligand.
DRAFT 2 April 2004
62
IR-9.3.5.2
Priority numbers
tio
n
s
The procedure for assigning priorities in mononuclear coordination systems is based on the
standard sequence rules developed for enantiomeric carbon compounds by Cahn, Ingold and
Prelog.14 (See also Section P-91 of Ref. 1). These CIP rules can be used quite generally for
da
assigning priorities to groups attached to a central atom.
om
me
n
The essence of these rules, when applied to coordination compounds, is that the ligands
attached to the central atom are compared to one another, beginning with the donor atom and
then moving outwards in the structure. The comparison is made on the basis of atomic
number and then, if required (e.g. when isotopes are being specified), atomic mass. Other
properties may be used for subsequent comparisons, but the need for them is sufficiently
rare that they need not be detailed here.
Once the ligands have been compared, the priority numbers are assigned as follows:
ec
(i) identical ligands are assigned the same rank,
na
lR
(ii) the ligand(s) with highest priority is(are) assigned the priority number 1;
those with the next highest priority, 2; and so on.
PA
C
Pr
ov
isi
o
Examples:
1.
5
1
O
C
2
Br
Cl
3
3
Ph3P
PPh3
NMe3
4
Priority sequence: Br > Cl > PPh3, PPh3 > NMe3 > CO
Priority numbers sequence: 1 > 2 > 3, 3 > 4 > 5
IU
2.
DRAFT 2 April 2004
Page 62 of 69
1
H 3N
OH
3
Pt
H 3N
2
N
CH3
N
tio
n
3
s
63
H
da
H
om
me
n
In Example 2, the heterocyclic ligand is given priority 2 since it has a lower atomic number
donor than OH, and the substitution of the nitrogen donor ranks it above the ammine ligands.
IU
PA
C
Pr
ov
isi
o
na
lR
ec
3.
Page 63 of 69
DRAFT 2 April 2004
64
CH3
..
N
_
_
_
H
4
N
N
3
N
CH3
6
MeEtHN
NHMe
3
NH2Me
H
_
_
M
..
N
Et
_
_
CH3
lR
5
na
M
..
N
isi
o
6
6
7
1
6
6
1
1
1
1
1
_
_
_
(6.5)
1 6
7
6
1
6
7
6
6
7
6
_
_
1
7
(7)
6 1
1 6
7 0
6
(6)
(6)
(6.5) 1
(6.5)
6
1
(6.5)
6
1
6
7
6
(6)
6
7
6
7
(7)
1
(6.5)
6
1
6
Pr
N
(6.5)
1 6
6
1
1
1
1
1
1
(6)
M
..
N
1
1
1
1
1
1
1
1
1
1
(6.5)
6 1
(6) 6
6 (6)
6
1
1
6.5 1
3
1
(6)
2
ov
7
1
1
1
1
1
1
1
NHMe2
M
1
da
6
CH3
4
2
7
1
1
1
1
ec
N
7
CH3
M
..
H
1
_
_
_
N
H
1
0
M
..
H
6
7
om
me
n
5
6
0
1
7
2
tio
n
6
M
..
6
1
1
1
1
s
Steps
Priority
sequence
IU
PA
C
In Example 3, all the ligating atoms are nitrogen atoms. The key illustrates how proceeding
along the branches of the ligand constituents allows priorities to be assigned. The numbers in
columns 1, 2 and 3 on the right are the atomic numbers of the atoms in the structures, with
those in brackets being used to take account of the presence of multiple bonds. The
averaging techniques used in the case of resonance structures (last two ligands in the list) are
given in the original paper.14
IR-9.3.5.3
Priming convention
DRAFT 2 April 2004
Page 64 of 69
65
da
tio
n
s
The priming convention is required in order to avoid ambiguity when using the configuration
index to describe the stereochemistry of systems that contain either more than one
polydentate ligand of a particular kind, or a polydentate ligand that contains more than one
coordinating fragment of a particular kind. This situation is found commonly with
bis(tridentate) complexes, but also arises in more complicated cases. The need for this
convention is best illustrated by example.
om
me
n
Bis(tridentate) complexes (i.e. octahedral complexes containing two identical linear tridentate
ligands) may exist in three stereoisomeric forms, and there will be more if the tridentate
ligands do not themselves contain some symmetry elements. The three isomers of the
simplest case are represented below (Examples 1, 2 and 3), along with their polyhedral
symbols (Section IR-9.3.2.1) and configuration indexes (Section IR-9.3.3.4). Complexes of
N-(2-aminoethyl)ethane-1,2-diamine or iminodiacetate can be described by these diagrams.
O
H
N
NH2
ec
H 2N
lR
N-(2-aminoethyl)ethane-1,2-diamine, or
N,N''-ethane-1,2-diylbis(ethane-1,2-diamine)
O
H
N
-O
O-
iminodiacetate, or
2,2'-azanediyldiacetate
ov
isi
o
na
The need for the priming convention can be seen by considering what the configuration
indexes of Examples 1 and 3 would be in the absence of the priming convention. The two
ligands are identical and consist of two similar fragments fused together. If the primes are
ignored, the two complexes have the same distributions of ligating atoms (four donors of
priority 1 in a square plane, and two of priority 2 trans to one another). They would therefore
have the same configuration index, even though they are clearly different complexes.
PA
C
Pr
One way to highlight the difference between these two examples is to note that, in Example 1,
all the donor atoms are trans to donors that are part of the other ligand. This is not true in
Example 3. Using primes to indicate the groupings of donor atoms in particular ligands
allows these two stereoisomers to be distinguished from one another by their configuration
indexes.
IU
Examples:
Page 65 of 69
DRAFT 2 April 2004
66
2
1'
2
1
1'
1
1'
1
2'
1
2'
1
2'
tio
n
s
1'
1
1'
da
1'
2. OC-6-2'1'
3. OC-6-11'
om
me
n
1. OC-6-1'1'
2
The priority numbers on one of the ligands are arbitrarily primed. The primed number is
assigned lower priority than the corresponding unprimed number, but a higher priority than
the next higher unprimed number. Thus 1' has lower priority than 1, but higher than 2.
lR
ec
The technique also distinguishes between stereoisomers for complexes of higher polydentate
ligands as indicated in Examples 4, 5 and 6 for linear tetradentate ligands such as N,N'-bis(2aminoethyl)ethane-1,2-diamine. In this case, the donor atom priority numbers in half of the
tetradentate ligand have been primed.
H 2N
H
N
NH2
na
N
H
Examples:
isi
o
N,N'-bis(2-aminoethyl)ethane-1,2-diamine
3
Pr
ov
1
2
PA
C
3
IU
4. OC-6-2'2
1
1
1'
3
1'
3
3
2'
2
2'
2
2'
3
5. OC-6-32
1'
6. OC-6-1'3
Pentadentate and hexadentate ligands can be treated similarly, as shown in Examples 7 to 10.
Examples 7 and 8 apply to stereoisomers of classic linear hexadentate ligands, whereas
Examples 9 and 10 apply to ligands containing a branched structure.
Examples:
DRAFT 2 April 2004
Page 66 of 69
67
3'
1
2'
2
2
1'
3
2'
tio
n
s
3
3'
1
da
1'
7. OC-6-3'3
8. OC-6-1'3'
1
2
1
5
4
3
om
me
n
1
3
1
5
4
10. OC-6-52
lR
9. OC-6-53
ec
2
isi
o
na
Example 11 illustrates the use of priming for assigning absolute configuration in a nonoctahedral structure. The chirality designation is determined by the system of assigning
primes to ligands. Specifically, the symbol on the top face is placed above the symbol 1" on
the bottom face. This produces the sequence shown and the chirality symbol C. The
stereochemical descriptor is TPR-6-1"11'-C.
ov
Example:
11.
Pr
PA
C
IU
IR-9.4
Page 67 of 69
3-
1"
1'
O
1
CH2
O
1'
1
Ho
O
O
CH2
1"
O
FINAL REMARKS
DRAFT 2 April 2004
3
68
REFERENCES
4.
5.
PA
C
10.
ov
8.
9.
Pr
7.
isi
o
na
6.
om
me
n
3.
New Blue Book.
In Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell
Scientific Publications, Oxford, 1990, the term didentate was used rather than bidentate,
for reasons of linguistic consistency. Reversion to the previously accepted term,
bidentate, reflects common usage.
For consistency, halide ligands are named fluorido, chlorido, bromido and iodido
throughout this book. Similarly, coordinated cyanide is named cyanido. The names
chloro, cyano, etc. are alternatives.
The names of the other hydrogen isotopes are discussed in Section IR-3.3.2.
The names of organic ligands should be assigned in accordance with IUPAC
recommendations, see Ref. 1.
In order to simplify the rules and to resolve ambiguities that may arise when it is not
clear whether a ligand is charged or not, the charge on a ligand is no longer considered
in determining ligand order in the formulae of coordination compounds. (In
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific
Publications, Oxford, 1990, anionic ligands were listed before neutral ligands).
Chapter II-7 of Nomenclature of Inorganic Chemistry II, Recommendations 2000,
Royal Society of Chemistry, 2001.
J. B. Casey, W.J. Evans and W.H. Powell, Inorg. Chem., 20, 1333 (1981).
Chapter II-5 of Nomenclature of Inorganic Chemistry II, Recommendations 2000,
Royal Society of Chemistry, 2001.
A. von Zelewski, Stereochemistry of Coordination Compounds, John Wiley & Sons,
Chichester, 1996.
A.M. Sargeson and G.A. Searle, Inorg. Chem., 4, 45 (1965); P.J. Garnett, D.W. Watts
and J.I. Legg, Inorg. Chem., 8, 2534 (1969); P.F. Coleman, J.I. Legg and J. Steele,
Inorg. Chem., 9, 937 (1970)
B. Bosnich, C.K. Poon and M.L. Tobe, Inorg. Chem., 4, 1102 (1965); P. O. Whimp,
M.F. Bailey and N.F. Curtis, J. Chem. Soc., 1956 (1970).
R.M. Hartshorn and D.A. House, J. Chem. Soc., Dalton Trans., 2577 (1998).
ec
1.
2.
lR
IR-9.5
da
tio
n
s
This chapter has described the means by which coordination compounds can be named and
formulated. These processes involve firstly identifying the central atom(s) and ligands (by
name, formula or abbreviation, depending on the context), and secondly defining the nature
of the attachment between the central atom(s) and the ligands. This latter step requires that
the coordinating atoms in the ligand be identified (if there is any ambiguity), and that the
spatial relationships between the ligands be described.
IU
11.
12.
13.
DRAFT 2 April 2004
Page 68 of 69
69
s
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
15.
16.
R.S. Cahn, C. Ingold and V. Prelog, Angew. Chem., Int. Ed. Engl., 5, 385 (1966); V.
Prelog and G. Helmchen, Angew. Chem., Int. Ed. Engl., 21, 567 (1982).
M.F. Brown, B.R. Cook and T.E. Sloan, Inorg. Chem., 7, 1563 (1978).
M. Brorson, T. Damhus and C.E. Schaeffer, Inorg. Chem., 22, 1569 (1983).
tio
n
14.
Page 69 of 69
DRAFT 2 April 2004
1
IR-10
Organometallic Compounds (Draft March 2004)
tio
n
s
CONTENTS
om
me
n
da
IR-10.1 Introduction
IR-10.2 Nomenclature of organometallic compounds of the transition metals
IR-10.2.1 Concepts and conventions
IR-10.2.1.1 Coordination number
IR-10.2.1.2 Chelation
IR-10.2.1.3 Specifying connectivity
IR-10.2.1.4 Oxidation number and net charge
IR-10.2.1.5 Valence-electron numbers and the 18-valence-electron
rule
IR-10.2.2 Compounds with one metal-carbon single bond
IR-10.2.3 Compounds with several metal-carbon single bonds from one ligand
IR-10.2.3.1 The mu (µ) convention
ec
IR-10.2.3.2 Chelating ligands
IR-10.2.3.3 The kappa (κ) convention
na
lR
IR-10.2.3.4 Bridging ligands
IR-10.2.3.5 Metal-metal bonding
IR-10.2.4 Compounds with metal-carbon multiple bonds
IR-10.2.5 Compounds with bonds to unsaturated molecules or groups
IR-10.2.5.1 The eta (η) convention
PA
C
Pr
ov
isi
o
IR-10.2.6 Metallocene nomenclature
IR-10.3 Nomenclature of organometallic compounds of the main group elements
IR-10.3.1 Introduction
IR-10.3.2 Organometallic compounds of groups 1 and 2
IR-10.3.3 Organometallic compounds of groups 13-16
IR-10.3.4 Priority order for central atoms in organometallic compounds
IR-10.3.4.1 Central atoms from groups 1-12 only
IR-10.3.4.2 Central atoms from both groups 1-12 and groups 13-16
IR-10.3.4.3 Central atoms from groups 13-16 only
IR-10.4 References
IU
IR-10.1
Page 1 of 48
INTRODUCTION
The enormous growth in organometallic chemistry over the last fifty years and the discovery
of new classes of compounds with unprecedented bonding modes has resulted in the need for
additional nomenclature rules for organometallic compounds. This Chapter is therefore
DRAFT 2 April 2004
2
tio
n
s
considerably expanded over Section I-10.9 of Ref. 1 and is largely based on the IUPAC
recommendations published in 1999 for organometallic compounds of the transition
elements.2
om
me
n
da
An organometallic compound is defined as any chemical species containing at least one bond
between a metal and a carbon atom in an organic molecule, ion or substituent group. The
names of organometallic compounds should therefore accord with the rules of both organic
and coordination chemistry nomenclature (even though these have tended to evolve
separately).
ec
The major part of this Chapter presents a system of nomenclature for transition metal
organometallic compounds, based on the additive nomenclature system (Section IR-4.3.4 and
Chapter IR-7) that is applied to coordination compounds (Chapter IR-9) but incorporating, as
far as possible, the rules for naming organic groups and substituents.3 In addition, further
rules are formulated which unambiguously designate the special modes of bonding often
found in organometallic compounds.
na
lR
The latter part of this Chapter briefly describes aspects of the naming of main group
organometallic compounds, where the substitutive system of nomenclature (Section IR-4.3.3
and Chapter IR-6) is applied by substituting the appropriate parent hydrides of the elements
of groups 13-16. The names of organometallic compounds of the groups 1 and 2 elements
are, on the other hand, based on the additive nomenclature system.
Pr
NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS OF THE TRANSITION
METALS
PA
C
IR-10.2.
ov
isi
o
It should be emphasized that the aim of nomenclature is confined to the precise description of
the composition of a compound and the connectivity of atoms within a molecule or ion. It is
particularly true of organometallic chemistry that nomenclature should not attempt to convey
details about the polarity of bonds, patterns of reactivity or methods of synthesis.
IU
IR-10.2.1
Concepts and conventions
The (additive) nomenclature of coordination complexes, the general definitions and rules of
which are given in Sections IR-9.1 and IR-9.2, provides the basis for the system presented
here for naming organometallic compounds of the transition elements. The general concepts
of coordination chemistry can be applied to organometallic compounds but need to be
expanded to deal with the additional modes of connectivity afforded by the interaction of
metals with, for example, organic ligands containing unsaturated groupings, such as alkenes,
DRAFT 2 April 2004
Page 2 of 48
3
IR-10.2.1.1
tio
n
s
alkynes and aromatic compounds. This section examines relevant concepts and conventions
from coordination chemistry as they are applied to organometallic compounds, and indicates
what new conventions need to be introduced in order to designate unambiguously the special
bonding modes of organometallic compounds.
Coordination number
da
The definition of coordination number as being equal to the number of σ-bonds between the
ligands and the central atom (Section IR-9.1.2.6) also applies to ligands such as CN−, CO,
om
me
n
N2 and PPh3, where the bonding of a single ligating atom to a metal may involve a
combination of σ- and π-components. The π-bond components are not considered in
determining the coordination number, and so [IrCl(CO)(PPh3)2], [RhI2(Me)(PPh3)2] and
[W(CO)6] have coordination numbers of four, five and six, respectively.
ec
However, this definition cannot be applied to the many organometallic compounds in which
two or more adjacent atoms of a ligand interact with the central metal atom through what is
often a combination of σ, π and δ bonding (the labels σ, π or δ referring to the symmetry of
lR
the orbital interactions between ligand and central atom).
Chelation
PA
C
IR-10.2.1.2
Pr
ov
isi
o
na
For example, a ligand such as ethene, consisting of two ligating carbon atoms, nevertheless
brings only one pair of electrons to the central atom. Likewise, ethyne, coordinating via both
carbon atoms, can be thought to bring either one or two pairs of electrons to a single metal
atom, depending on the type of coordination involved. Both ligands are normally regarded as
monodentate. This changes when ethene or ethyne is considered to add oxidatively to a
central metal atom; they are then considered to be bidentate chelating ligands which, on
electron counting and dissection of the coordination entity to determine oxidation numbers,
are assumed to take two pairs of electrons with them. This different view can be expressed by
referring to compounds of such ligands as metallacyclopropanes or metallacyclopropenes
rather than alkene or alkyne complexes.
IU
The concept of chelation (Section IR-9.1.2.7) can again be applied strictly only to those
organometallic complexes in which the donor atoms of a ligand are attached to the central
metal atom through σ-bonds alone. Otherwise, ambiguities will result, as outlined above, even
Page 3 of 48
with a simple ligand such as ethene. Butadiene and benzene supply two and three pairs of
electrons upon coordination and are therefore regarded as bi- and tridentate ligands,
respectively. In stereochemistry, however, such ligands are often treated as if they were
monodentate.
DRAFT 2 April 2004
4
Specifying connectivity
s
IR-10.2.1.3
tio
n
In the event of a ligand containing several different donor atoms, particularly when not all are
used, the point or points of attachment to the metal are specified using the kappa (κ)
om
me
n
da
convention (see Sections IR-9.2.4.1 and IR-9.2.4.2). In organometallic nomenclature the
ligating carbon atoms are usually sufficiently specified by the locants preceding the
appropriate suffix within the ligand name, but use of the kappa notation becomes necessary to
indicate the attachment of heteroatoms, and also to specify the particular points of attachment
of a single ligand when bridging different metal centres in a polynuclear complex. The
strength of the kappa convention is that its use completely avoids any ambiguities in
describing the connectivities between a ligand and one or more metal centres. Its use in
organometallic nomenclature is discussed further in Section IR-10.2.3.3.
A complementary notation, the eta (η) convention, is used to specify the number ('hapticity')
lR
ec
of contiguous ligating atoms that are involved in bonding to one or more metals. The need for
this convention arises from the special nature of the bonding of unsaturated hydrocarbons to
metals via their π-electrons, and is required only when there are several contiguous atoms
involved in the bond to the metal. The contiguous atoms of the π-coordinated ligand are often
isi
o
na
the same element, but they need not be, and they may also be atoms other than carbon. The
eta convention is defined in Section IR-10.2.5.1, where its use is extensively illustrated (see
also Section IR-9.2.4.3). While complicated structures will require use of both the kappa and
eta conventions, these descriptors are complementary and should not be interchanged in
usage.
Pr
ov
Organic ligands with the ability to form more than one bond to a metal centre may be either
chelating (if bonding to a single metal), bridging (if bonding to more than one metal), or
sometimes even both chelating and bridging. As in coordination chemistry, the bridging
bonding mode is indicated by the Greek letter µ (mu) prefixing the ligand name (Section IR-
PA
C
9.2.5.2). This convention is further exemplified for organometallic compounds in Sections
IR-10.2.3.1 and IR-10.2.3.4.
IU
IR-10.2.1.4
Oxidation number and net charge
The concept of oxidation number (see also Sections IR-4.6.1, IR-5.4.2.2 and IR-9.1.2.8)
follows from the application of the classical definition of coordination number and is
therefore difficult to apply to organometallic compounds where often the coordination
number cannot be unequivocally assigned. This is especially true when it cannot be
determined whether complexation by a ligand is better regarded as a Lewis-acid or -base
DRAFT 2 April 2004
Page 4 of 48
5
s
association or as an oxidative addition. In such ambiguous cases, the net charge on the
coordination entity is preferred in most nomenclature practices.
Valence-electron numbers and the 18-valence-electron rule
om
me
n
IR-10.2.1.5
da
tio
n
Thus, no formal oxidation numbers will be attributed to the central metal atoms in the
following sections. This does not mean, however, that the oxidation state of a metal or a
ligand is unimportant when discussing reaction mechanisms, the polarity of bonds or the
results of spectroscopic or structural studies. Oxidation numbers also have to be assigned, if
only arbitrarily, when establishing the number of valence electrons.
While formal oxidation numbers will not be assigned in organometallic complexes in the
following sections, it is nevertheless important to establish the number of valence electrons
associated with each complex as well as the net charge.
na
lR
ec
The 18-electron rule is based on the valence-bond formalism of localised metal-ligand bonds;
it states that thermodynamically stable transition-metal organometallic compounds are formed
when the sum of the number of metal d electrons plus the number of electrons conventionally
regarded as supplied by the ligands equals 18. In this way, the metal formally attains the
electron configuration of the next higher noble gas.
isi
o
Table IR-10.1 lists ligands commonly encountered in organotransition metal compounds
together with the numbers of electrons they are considered to supply. The oxidation number
of the metal has to be adjusted in relation to the charge attributed to the various ligands to
obtain the correct net charge.
ov
Table IR-10.1 Number of bonding electrons of commonly encountered ligands
Neutral
1
Positive
-
Negative
2
η2-alkene, CO, CS, amine, nitrile,
isocyanide, phosphane
2
-
-
alkylidene (CR2) or alkanediide (CR22−)
2
-
4 (2–)
azanylidene (nitrene, NR)
azanediide, (imide, NR2−)
2
-
4 (2–)
oxide O2−
-
-
4 (2–)
alkylidyne (CR) or alkanetriide (CR3−)
3
-
6 (3–)
IU
PA
C
Pr
Ligand a
alkyl, aryl, hydride, halide, amide
Page 5 of 48
DRAFT 2 April 2004
6
3
-
4
NO (bent)
1
-
-
NO (linear)b
3
2
-
η4-diene, e.g. η4-cyclobutadiene
4
-
η5-cyclopentadienyl
5
-
η6-arene, η6-triene
6
η8-cyclooctatetraened
a The η (eta) notation is described in
tio
n
-
6
da
-
om
me
n
η7-tropyliumc or η7-cycloheptatrienyl
s
η3-allyl, η3-enyl, e.g. η3-cyclopropenyl
-
7
6
-
8
-
10 (2–)
Section IR-10.2.5.1. b NO+ is isoelectronic
with CO and as such acts as a two-electron ligand. c The name 'tropylium'
designates the monocation C7H7+. d The coordinated C8H8 ligand may also be
ec
regarded as a dianion.
lR
When determining valence electron numbers, several conventions should be taken into
account.
PPh3
Cl
Cl
IU
PA
C
Pr
ov
Example:
1.
isi
o
na
(a) The intramolecular partitioning of electrons is made so as to ensure that the total charge
on the complex remains unchanged.
Ru
CH2
PPh3
2 PPh3
4 e– or
2 PPh3
4 e–
2 Cl
1 :CH2
2 e–
2 e–
2 Cl–
1 CH22–
4 e–
4 e–
1 Ru0
8 e–
1 RuIV
4 e–
Total
16 e–
16 e–
(b) A metal-metal bond contributes one electron to the count for each metal. Metal-metal
double or triple bonds supply two and three electrons, respectively, to each metal.
DRAFT 2 April 2004
Page 6 of 48
7
Mn
Mn
OC
CO
CO
CO
CO
CO
da
OC
CO
OC
tio
n
OC
s
Example:
2.
om
me
n
5 CO
10 e–
Mn0
7 e–
Mn—Mn 1 e–
18 e–
Total
ec
(c) The electron pair of a bridging ligand such as CO donates one electron to each of the
bridged metals.
na
lR
Example:
3.
O
C
Re
IU
PA
C
Pr
ov
isi
o
Re
IR-10.2.2
Page 7 of 48
C
O
1 C5Me5-
C
O
3 (µ-CO)
6 e–
3 e–
ReI
Re≡Re
6 e–
3 e–
Total
18 e–
For organometallic compounds of the f-block elements (lanthanoids and actinoids) this
procedure is not applicable. Exceptions to the 18-electron rule are also found with the early
and late-transition metals (see Example 1 above) and in complexes in which the central atom
has a high formal oxidation state, e.g. [WMe6] or [ReMe(O)3].
Compounds with one metal-carbon single bond
DRAFT 2 April 2004
8
tio
n
s
In naming organometallic compounds the usual rules for naming ligands in coordination
entities are applied if the ligand is organic but coordinates via an atom other than carbon
−
−
(Section IR-9.2.2.3). Thus, the ligand MeCOO is named acetato, Me2N is named
dimethylamido (or N-methylmethanaminido), and PPh3 is named triphenylphosphane.
om
me
n
da
If organic ligands coordinating via one carbon atom are regarded as anions formed by the
removal of one hydron from a carbon atom of an organic molecule, the anions are named by
replacing the final 'e' of the parent compound name by 'ide' (see Section P-72.2.2 of Ref. 3).
This rule is most commonly applied when using compositional nomenclature with highly
ionic organic compounds of the alkali and alkaline earth metals, such as sodium methanide or
potassium cyclopentadienide.
ec
As in the nomenclature of coordination compounds, the ending 'ide' is replaced by 'ido' upon
coordination of the ligand. All names must have locants, starting with propane (except
monocyclic, unsubstituted rings).
Examples:
_
Me
_
Et
lR
methanido
ethanido
prop-2-en-1-ido
na
(CH2=CHCH2)
_
Ph
_
(C5H5)
_
isi
o
benzenido
cyclopentadienido
The compound [TiCl3Me] would therefore be called trichlorido(methanido)titanium by the
ov
systematic application of the additive nomenclature of coordination compounds.
IU
PA
C
Pr
The alternative for naming an organic ligand attached via a single carbon atom is to regard it
as a substituent group, its name being derived from the parent hydride from which one
hydrogen atom has been removed. This designation is somewhat arbitrary as such ligands in
organometallic chemistry are generally treated as anions when deducing oxidation states,
although the bonding in reality may be highly covalent. However, this system of
nomenclature (see Section P-29.2 of Ref. 3) has a long tradition in organic and
organometallic chemistry, and its major advantage is that names used in common practice for
organic groups can be applied unchanged.
In this system suffixes are used according to two methods:
DRAFT 2 April 2004
Page 8 of 48
9
da
methyl
ethyl
cyclohexyl
1-methylbutyl
trimethylsilyl
om
me
n
Examples:
CH3–
CH3CH2–
C6H11–
CH3CH2CH2C(Me)H–
Me3Si–
tio
n
s
(a) The suffix 'yl' replaces the ending 'ane' of the parent hydride name. The atom with the free
valence terminates the chain and always has the locant '1', which is omitted from the name.
This method is best employed in simple cases like saturated acyclic and monocyclic
hydrocarbon substituent groups and for the mononuclear parent hydrides of silicon,
germanium, tin and lead. (See also Section P-29.2.1 of Ref. 3).
Thus the compound [TiCl3Me] would be called trichlorido(methyl)titanium by this method.
na
lR
ec
(b) In a more general method, the suffix 'yl' is added to the name of the parent hydride with
elision of the terminal 'e', if present. The atom with the free valence is given a number as low
as is consistent with any established numbering of the parent hydride. The locant number,
including '1', must always be cited. (See Section P-29.2.2 of Ref. 3 for a more complete
discussion of substituent prefix names).
pentan-1-yl
pentan-2-yl
ov
isi
o
Examples:
CH3CH2CH2CH2CH2–
CH3CH2CH2C(Me)H–
cyclohexan-1-yl
CH2=CHCH2–
prop-2-en-1-yl
PA
C
Pr
H
IU
In fused polycyclic hydrocarbons as well as in heterocyclic systems, special numbering
schemes are adopted. (See Ref. 4 and Sections P-29.3.3 and P-29.3.4 of Ref. 3). The locant
of the ligating atom is then also indicated before the ending 'yl'.
Page 9 of 48
Examples:
DRAFT 2 April 2004
H
s
10
tio
n
inden-1-yl
N
H
om
me
n
morpholin-2-yl
da
H
O
Table IR-10.2 gives the names used for ligands forming a single bond to a metal, and this is
followed by examples illustrating the naming of compounds containing one metal-carbon
single bond.
lR
ec
In Table IR-10.2 (as well as Tables IR-10.3 and IR-10.5) the organic ligands are listed by
their systematic additive and systematic substitutive names. In all the Tables an alternative
name is sometimes included that is generally preferred either for historical reasons or for
reasons of brevity.
na
Table IR-10.2 Names for ligands forming a metal-carbon single bond
Systematic additive
name
isi
o
Ligand formula
H3C
methyl
ethanido
ethyl
Alternative name
propan-1-ido
propyl
(CH3)2CH
propan-2-ido
propan-2-yl
isopropyl
prop-2-en-1-ido
prop-2-en-1-yl
allyl
butan-1-ido
butyl
butan-2-ido
butan-2-yl
or 1-methylpropyl
sec-butyl
2-methylpropan-1-ido
2-methylpropyl
isobutyl
Pr
CH3CH2CH2
PA
C
CH2=CHCH2
CH3CH2CH2CH2
CH3CH2
IU
methanido
ov
CH3CH2
Systematic substitutive
name
CH3
C
H
H3C
CH CH2
H3C
DRAFT 2 April 2004
Page 10 of 48
11
CH3
2-methylpropan-2-yl
or 1,1-dimethylethyl
CH3
CH3
2,2-dimethylpropan-1ido
C CH2
2,2-dimethylpropyl
om
me
n
CH3
H2C
cyclopropanido
CH
cyclopropyl
H2C
H2
C
cyclobutanido
CH
C
H2
cyclopentadienido
C6H5
benzenido
C6 H5CH2
phenylmethanido
phenylmethyl
benzyl
1-oxoethanido
ethanoyl
acetyl
1-oxopropanido
propanoyl
propionyl
1-oxobutanido
butanoyl
butyryl
oxo(phenyl)methanido
phenylcarbonyl
benzoyl
H2C CH
ethenido
ethenyl
vinyl
HC C
ethynido
ethynyl
H3Si
silanido
silyl
H3Ge
germanido
germyl
na
isi
o
O
O
Pr
C3H7 C
ov
O
C2H5 C
PA
C
O
IU
lR
C5H5
H3C C
Page 11 of 48
cyclobutyl
ec
H2C
neopentyl
da
H3C
tert-butyl
s
2-methylpropan-2-ido
C
tio
n
H3C
C
cyclopentadienyl
phenyl
DRAFT 2 April 2004
12
stannanido
[OsEt(NH3)5]Cl
2.
da
pentaammine(ethyl)osmium(1+) chloride
Li[CuMe2]
om
me
n
lithium dimethylcuprate(1−)
3.
CrR =
CrR4
tio
n
Examples:
1.
stannyl
s
H3Sn
1
ec
Cr
[Pt{C(O)Me}Me(PEt3)2]
na
4.
lR
tetrakis(bicyclo[2.2.1]heptan-1-yl)chromium
Pr
ov
5.
isi
o
acetyl(methyl)bis(triethylphosphane)platinum
Me
Fe
Ph
OC
Ph3P
Me
IU
PA
C
carbonyl(η5-cyclopentadienyl)[(2E)3-phenylbut-2-en-2-yl](triphenylphosphane)iron
6.
Ph3P
py
Rh
Ph3P
C
CPh
(phenylethynyl)(pyridine)bis(triphenylphosphane)rhodium
DRAFT 2 April 2004
Page 12 of 48
13
7.
P
H
s
P
tio
n
Ru
P
om
me
n
da
P
bis[ethane-1,2-diylbis(dimethylphosphane)-κ2P]hydrido(naphthalen-2-yl)ruthenium
P = Me2PCH2CH2PMe2 = ethane-1,2-diylbis(dimethylphosphane) = dmpe
P
IR-10.2.3
Compounds with several metal-carbon single bonds from one ligand
ov
isi
o
na
lR
ec
When an organic ligand forms more than one metal-carbon single bond (to one or more
metal atoms), the ligand name is derived from the parent hydrocarbon from which the
appropriate number of hydrogen atoms have been removed. In the systematic substitutive
name, the suffix 'diyl' or 'triyl' is attached to the name of the parent hydrocarbon if two or
three hydrogen atoms, respectively, are replaced by one or more metal atoms. There is no
removal of the terminal 'e', such elision being required only before suffixes beginning with 'a',
'i', 'o', 'u' or 'y'. The locant number must always be cited, except for ligands derived from
methane. Alternatively, when using additive nomenclature, the endings 'diido' and 'triido'
should be used. This nomenclature also applies to hypervalent coordination modes, e.g. for
bridging methyl groups. Typical ligands forming two or three metal-carbon single bonds are
listed in Table IR-10.3.
Pr
Table IR-10.3 Names for ligands forming several metal-carbon single bonds
Systematic additive
name
Systematic substitutive
name
Alternative
name
–CH2–
methanediido
methanediyl
methylene
–CH2CH2–
ethane-1,2-diido
ethane-1,2-diyl
ethylene
–CH2CH2CH2–
propane-1,3-diido
propane-1,3-diyl
–CH2CH2CH2CH2–
butane-1,4-diido
butane-1,4-diyl
HC
methanetriido
methanetriyl
IU
PA
C
Ligand formula
Page 13 of 48
DRAFT 2 April 2004
CH3C
ethane-1,1,1-triido
ethane-1,1,1-triyl
–CH=CH–
ethene-1,2-diido
ethene-1,2-diyl
H2C C
ethene-1,1-diido
ethene-1,1-diyl
–C≡C–
ethyne-1,2-diido
ethyne-1,2-diyl
tio
n
ethane-1,1-diyl
da
ethane-1,1-diido
om
me
n
IR-10.2.3.1
CH3HC
s
14
The mu (µ) convention
Organic ligands forming more than one metal-carbon bond can be either chelating, if
coordinating to one metal atom, or bridging, if coordinating to two or more metal atoms. A
bridging bonding mode is indicated by the Greek letter µ (Sections IR-9.2.5.2 and IR-
ec
10.2.3.4).
H2
C
CH2
na
H 2C
lR
Example:
1.
H2
C
H 2C
CH2
M
M
isi
o
M
µ-propane-1,3-diyl
(bridging)
ov
propane-1,3-diyl
(chelating)
Pr
The number of metal atoms connected by a bridging ligand is indicated by a right subscript,
µn, where n ≥ 2, though the bridging index 2 is not normally indicated.
IU
PA
C
Example:
2.
H3
C
H3
C
M
M
µ-methyl
M
M
M
µ3-methyl
DRAFT 2 April 2004
Page 14 of 48
15
The name methylene for CH2 can only be used in connection with a bridging bonding mode
(µ-methylene), whereas a CH2 ligand bonding to one metal only has a metal-carbon double
tio
n
s
bond and should be named as methylidene (see Section IR-10.2.4).
H2
C
M
M
M
µ-methylene
CH2
om
me
n
methylidene
da
Example:
3.
Likewise, the ligand HC will have at least three different bonding modes: bridging three
metals (µ3-methanetriyl), bridging two metals (µ-methanylylidene) and coordinating to one
metal (methylidyne, see Section IR-10.2.4).
H
C
H
C
M
M
lR
M
ec
Example:
4.
na
µ3-methanetriyl
M
M
µ-methanylylidene
M
CH
methylidyne
isi
o
In a bridging mode the ligand CH2CH2 should be called µ-ethane-1,2-diyl, while the same
ligand coordinating through both carbon atoms to a single metal centre should be called η2-
ov
ethene (see Section IR-10.2.5).
IU
PA
C
Pr
Example:
5.
Page 15 of 48
H2
C
M
H2
C
H 2C
CH2
M
M
µ-ethane-1,2-diyl
η2-ethene
A similar situation arises with CHCH which, when bridging with the carbon atoms
individually bonded to each of two metals, should be called µ-ethene-1,2-diyl or, when the
metal-carbon bonds are double, µ-ethanediylidene (see Section IR-10.2.4). The same ligand
coordinating through both carbon atoms to both metal centres should be called µ-ethyne;
DRAFT 2 April 2004
16
when coordinated through both carbons to one metal it is named η2-ethyne (see Section IR-
s
10.2.5).
M
M
µ-ethene-1,2-diyl
HC
M
M
M
µ-ethanediylidene
HC
CH
M
CH
M
µ-ethyne
ec
η2-ethyne
Chelating ligands
lR
IR-10.2.3.2
H
C
da
H
C
H
C
om
me
n
H
C
tio
n
Example:
6.
na
Where a ligand is chelating, the coordinating atoms should be indicated either by specifying
them within the ligand name (such as propane-1,3-diyl) or by applying the italicized donor
atom symbols of the κ notation (propanediyl-κ2C1,C3) (Sections IR-9.2.4.2 and IR-
isi
o
10.2.3.3). For ligands coordinating only via carbon atoms, it is generally preferable to specify
the ligating atoms within the ligand name and not with the κ-notation. The locant '1' is
Pr
ov
assigned so as to create the longest chain of carbon atoms, and the direction of numbering is
chosen to give the lowest possible locants to side chains or substituents. For heterocyclic and
polycyclic ligand systems special numbering schemes for the organic moieties are employed
(see Ref. 4 and Sections P-29.3.3 and P-29.3.4 of Ref. 3). Note that an alternative
nomenclature for metallacycles, e.g. Examples 1, 2 and 3, is currently being developed.
IU
PA
C
Examples:
1.
H 2C
H2
C
PPh3
Pt
H 2C
C
H2
PPh3
(butane-1,4-diyl)bis(triphenylphosphane)platinum
DRAFT 2 April 2004
Page 16 of 48
17
2.
s
+
Me
H
H
da
Me
tio
n
Ir(PEt3)3
3.
Ph
Ph
3
Ph3P
om
me
n
(2,4-dimethylpenta-1,3-diene-1,5-diyl)tris(triethylphosphane)iridium(1+)
Pt
2
C
1
O
ec
PPh3
The kappa (κ) convention
na
IR-10.2.3.3
lR
(1-oxo-2,3-diphenylpropane-1,3-diyl)bis(triphenylphosphane)platinum
isi
o
Chelate rings that contain a coordinate (dative) bond from a heteroatom in addition to a
carbon attachment should be named using the κ convention. In this notation (see Section IR-
ov
9.2.4.2) the coordinating atoms of a polydentate ligand bonding to a metal centre are
indicated by the Greek letter kappa, κ, preceding the italicized element symbol of each ligating
atom. A right superscript numeral may be added to the symbol κ to indicate the number of
Pr
identically bound ligating atoms; non-equivalent ligating atoms should each be indicated by
an italicized element symbol preceded by κ.
IU
PA
C
In simple cases one or more superscript primes on the element symbol may be used to
differentiate between donor atoms of the same element. Otherwise a right superscript numeral
corresponding to the conventional numbering of the atoms in the ligand is used to define
unambiguously the identity of the ligating atom. These symbols are placed after that portion
of the ligand name which represents the particular functionality, substituent group, ring or
chain in which the ligating atom is found.
Often it is only necessary for the coordinating heteroatom to be specified using the κ
convention, the ligating carbon atom being adequately specified by the appropriate
Page 17 of 48
DRAFT 2 April 2004
18
substitutive suffix. For illustrative purposes only, an arrow is used in the examples that follow
to indicate a coordinate bond in the chelate ring. In Example 1 the κC1 specification is
tio
n
s
included for clarity but is not strictly necessary as the ligating phenyl carbon atom, being
bonded to the metal, is automatically labelled '1'.
2
da
Examples:
1.
N
om
me
n
N
1
Mn
(CO)4
tetracarbonyl[2-(phenyldiazenyl-κN2)phenyl-κC1]manganese
2.
1
ec
H
Pri3P
Rh
3
O
lR
Cl
2
4
na
PPri3
IR-10.2.3.4
isi
o
chloridohydrido(2-methyl-3-oxo-κO-but-1-en-1-yl)bis(triisopropylphosphane)rhodium
Bridging ligands
ov
Bridging ligands are indicated by the Greek letter µ (mu) prefixing the ligand name (see
Pr
Sections IR-9.2.5.2 and IR-10.2.3.1). Bridging ligands are cited before terminal ligands, the
ligands in each category being listed in alphabetical order, and multiple bridging is listed in
descending order of complexity, e.g. µ3 bridging before µ2 bridging.
IU
PA
C
Example:
1.
Me
(OC)5Re
CH
Re(CO)5
(µ-ethane-1,1-diyl)bis(pentacarbonylrhenium)
DRAFT 2 April 2004
Page 18 of 48
19
tio
n
s
The metal centres in heterobinuclear molecules are numbered and listed according to the
element priority sequence given in Table VI*, the higher priority central atom being numbered
'1' and listed in the name first (see Sections IR-2.15.4 and IR-9.2.5.1).
The numerical locants of the central atoms are used in conjunction with the κ notation to
indicate the distribution of the ligating atoms. Such locants are placed before the κ symbol,
da
which, as before, may be followed by a right superscript numeral to denote the number of
equivalent ligating atoms bonded to the central atom specified by the locant (see Section IR9.2.5.5). Thus, decacarbonyl-1κ5C,2κ5C indicates that the carbon atoms of five carbonyl
om
me
n
ligands are bonded to central atom number 1 and another five to central atom number 2.
Where bridging is accomplished by different atoms of the same group, the ligating locants
and symbols are separated by a colon, e.g. µ-propane-1,2-diyl-1κC1:2κC2.
Example:
2.
3
1
(OC)5Re
ec
CH3
2
1
2
Mn(CO)5
lR
C
H2
Metal-metal bonding
isi
o
IR-10.2.3.5
na
decacarbonyl-1κ5C,2κ5C-(µ-propane-1,2-diyl-1κC1:2κC2)rheniummanganese
PA
C
Pr
ov
Metal-metal bonding is indicated by the italicized element symbols of the appropriate metal
atoms, separated by an 'em' dash and enclosed in parentheses, placed after the list of central
atoms and before the ionic charge. The element symbols are placed in the same order as the
central atoms appear in the name, i.e. with the higher priority element, as determined from
Table VI, given first. The number of such metal-metal bonds is indicated by an arabic
numeral placed before the first element symbol and separated from it by a space. For the
purpose of nomenclature, no distinction is made between different metal-metal bond orders.
IU
Examples:
1.
*
Page 19 of 48
H 2C
(OC)4Os
CH2
Os(CO)4
Tables numbered with a Roman numeral are collected together at the end of this book.
DRAFT 2 April 2004
20
(µ-ethane-1,2-diyl)bis(tetracarbonylosmium)(Os—Os)
s
2.
tio
n
Me
C
Co(CO)3
da
(OC)3Co
om
me
n
Co
(CO)3
(µ3-ethane-1,1,1-triyl)-triangulo-tris(tricarbonylcobalt)(3 Co—Co)
3.
O
C
1
W
Re
C
O
lR
ec
C
O
2
di-µ-carbonyl-carbonyl-2κC-tris[1,1,2(η5)-cyclopentadienyl]tungstenrhenium(W—Re)
IR-10.2.4
isi
o
na
For a more detailed discussion of binuclear compounds and larger polynuclear clusters, with
further examples, see Section IR-9.2.5.
Compounds with metal-carbon multiple bonds
Pr
ov
Ligands regarded as forming metal-carbon double or triple bonds are also given substituent
prefix names derived from the parent hydrides, the ligand names ending with 'ylidene' for a
double bond and with 'ylidyne' for a triple bond. These suffixes are used according to two
methods (see Sections P-29.2.1 and P-29.2.2 of Ref. 3).
IU
PA
C
(a) The suffix 'ylidene' or 'ylidyne' replaces the ending 'ane' of the parent hydride name. The
atom with the free valence terminates the chain and always has the locant '1', which is
omitted from the name. This method is recommended only for saturated acyclic and
monocyclic hydrocarbon substituent groups and for the mononuclear parent hydrides of
silicon, germanium, tin, lead and boron. Note that the suffix 'ylene' should only be used in
conjunction with µ to designate bridging –CH2– (methylene) or –C6H4– (phenylene) (see
Section IR-10.2.3.1).
DRAFT 2 April 2004
Page 20 of 48
21
Example:
1.
CH3CH2CH=
(a) propylidene, or
om
me
n
(b) propan-1-ylidene
da
tio
n
s
(b) In a more general method, the suffix 'ylidene' or 'ylidyne' is added to the name of the
parent hydride with elision of the teminal 'e', if present. The atom with the free valence is
given a number as low as is consistent with any established numbering of the parent
hydride. Except for ligands which are bonded to a metal in an unambiguous fashion or when
using the suffix 'ylidyne', the locant '1' must always be cited.
ec
Note that in numbering a ligand that has several points of attachment, the longest chain of
carbon atoms is chosen as the parent chain before assigning the lowest possible locant to
the atom with the free valence. In a metallacycle, the direction of numbering is chosen so as
to give the lowest possible locants to side chains or substituents. Once again, special
numbering schemes apply to heterocyclic and polycyclic systems (see Ref. 4 and Sections
P-29.3.3 and P-29.3.4 of Ref. 3).
isi
o
na
lR
In a ligand containing one or more metal-carbon single bonds as well as metal-carbon
multiple bonds, the order of endings is 'yl', 'ylidene', 'ylidyne'. Method (b) should then be
used to give the lowest possible set of locants for the free valencies. If a choice remains,
lower numbers are selected for the 'yl' positions before the 'ylidene' positions and then for
any side chains or substituents.
ov
Example:
2.
C
propan-1-yl-1-ylidene
Pr
CH3 CH2
PA
C
Typical ligands forming a metal-carbon double or triple bond are listed in Table IR-10.4,
and this is followed by examples illustrating the naming of compounds containing one or
more metal-carbon multiple bonds.
IU
Table IR-10.4 Names for ligands forming metal-carbon multiple bonds
Page 21 of 48
Ligand formula
Systematic name
H2C
methylidene
DRAFT 2 April 2004
Alternative name
ethylidene
H2C C
ethenylidene
vinylidene
H2C HC HC
prop-2-enylidene
allylidene
H2C C
propa-1,2-dienylidene
allenylidene
H3C
propan-2-ylidene
C
CH3
H3C
C
isopropylidene
om
me
n
H3C
da
C
tio
n
H3CCH
s
22
2,2-dimethylpropylidene
CH
CH3
cyclopropylidene
ec
C
lR
cyclobutylidene
na
C
cyclopenta-2,4-dien-1-ylidene
isi
o
C
phenylmethylidene
ov
PhHC
HC
Pr
HC
methylidyne
ethylidyne
EtC
propylidyne
PA
C
IU
methanylylidene
MeC
CH3
H3C
C
C
benzylidene
2,2-dimethylpropylidyne
CH3
PhC
phenylmethylidyne
DRAFT 2 April 2004
benzylidyne
Page 22 of 48
23
OMe
O
C
H
1
tio
n
2
s
Examples:
1.
CO
W NCMe
C
da
C
O C
O
om
me
n
trans-(acetonitrile)tetracarbonyl(2-methoxybenzylidene)tungsten
2.
Me
ec
Ir(PEt3)3
lR
Me
(2,4-dimethylpenta-1,3-dien-1-yl-5-ylidene)tris(triethylphosphane)iridium
isi
o
na
3.
Mn C
C
C
Me
ov
OC
OC
Me
Pr
dicarbonyl(η5-cyclopentadienyl)(3-methylbuta-1,2-dien-1-ylidene)manganese
IU
PA
C
4.
O
C
I
C
O
Cr
C
O
CNEt2
C
O
trans-tetracarbonyl[(diethylamino)methylidyne]iodidochromium
5.
Page 23 of 48
DRAFT 2 April 2004
24
CCMe3
P
CH2CMe3
s
W
CHCMe3
tio
n
P
[ethane-1,2-diylbis(dimethylphosphane)-κ2P](neopentyl)(neopentylidene)(neopentylidyne)tungsten
da
IR-10.2.5
P = Me2PCH2CH2PMe2 = ethane-1,2-diylbis(dimethylphosphane) = dmpe
Compounds with bonds to unsaturated molecules or groups
om
me
n
P
Since the discovery of Zeise's salt, K[Pt(η2-C2H4)Cl3], the first organometallic complex of
a transition metal, and particularly since the first reported synthesis of ferrocene, [Fe(η5C5H5)2], the number and variety of organometallic compounds with unsaturated organic
ligands has increased enormously.
lR
ec
Complexes containing ligands which coordinate to a central atom with at least two adjacent
atoms in a 'side-on' fashion require a special nomenclature. These ligands normally contain
groups that coordinate via the π-electrons of their multiple bonds, such as alkenes, alkynes
na
and aromatic compounds, but they may also be carbon-free entities containing bonds
between heteroelements; such compounds are generally referred to as 'π-complexes'.
However, the expression 'π-coordinated' is too imprecise, since the exact nature of the
bonding (σ, π, δ) is often uncertain. The atoms bonded to the metal atom are therefore
isi
o
indicated in a manner independent of theoretical implications. Thus, the use of the prefixes
σ and π is not recommended in nomenclature; these symbols refer to the symmetry of
ov
orbitals and their interactions, which are irrelevant for nomenclature purposes.
Pr
From the point of view of oxidation states, ligands such as alkenes, alkynes, nitriles,
diazenes, and others such as allyl (C3H5), butadiene (C4H6), cyclopentadienyl (C5H5),
cycloheptatrienyl (C7H7) and cyclooctatetraene (C8H8), may be formally regarded as
IU
PA
C
anionic, neutral or cationic (see Section IR-10.2.1.5 and Table IR-10.1). The structures of,
and bonding in, their complexes may also be complicated or ill-defined. Names for such
ligands are therefore chosen that indicate stoichiometric composition and are derived in a
similar way to those for the ligands discussed in preceding Sections.
Neutral ligands are given a name in which that part of the molecule that is attached to the
metal becomes the principal group. All other characteristic groups are then cited as
prefixes. Other ligands are given the substituent names ending in 'yl', 'diyl', 'ylidene', etc.,
depending on the number of hydrogen atoms removed and the type of bonding (as
DRAFT 2 April 2004
Page 24 of 48
25
IR-10.2.5.1
tio
n
s
discussed in Sections IR-10.2.2, IR-10.2.3 and IR-10.2.4). Alternatively, the endings 'ido',
'diido', etc. can be used. As before, a special nomenclature applies to fused polycyclic or
unsaturated heterocyclic ligands (see Ref. 4 and Sections P-29.3.3.and P-29.3.4 of Ref. 3).
The eta (η) convention
da
The special nature of the bonding of unsaturated hydrocarbons to metals via their π-
om
me
n
electrons has led to the development of the 'hapto' nomenclature to designate
unambiguously the unique bonding modes of the compounds so formed.5 (See also Section
IR-9.2.4.3). The Greek symbol η (eta) provides a topological description by indicating the
ec
connectivity between the ligand and the central atom. The number of contiguous atoms in
the ligand coordinated to the metal is indicated by a right superscript numeral, e.g. η3 ('eta
three' or 'trihapto'), η4 ('eta four' or 'tetrahapto'), η5 ('eta five' or 'pentahapto'), etc. The
symbol η is added as a prefix to the ligand name, or to that portion of the ligand name most
appropriate to indicate the connectivity, as in cyclopenta-2,4-dien-1-yl-η2-ethene versus
vinyl-η5-cyclopentadienyl:
lR
M
vinyl-η5-cyclopentadienyl
na
cyclopenta-2,4-dien-1-yl-η2-ethene
M
ov
isi
o
Complexes of unsaturated systems incorporating heteroatoms may be designated in the
same manner if both the carbon atoms and adjacent heteroatoms are coordinated. Names for
typical unsaturated molecules and groups acting as ligands are listed in Table IR-10.5, and
this is followed by examples illustrating the naming of compounds containing such ligands.
Systematic additive name
Systematic substitutive name
Alternative name
η3-propenido
η3-propenyl
η3-allyl
η3-(Z)-butenido
η3-(Z)-butenyl
η3-2-methylpropenido
η3-2-methylpropenyl
IU
PA
C
Ligand a
Pr
Table IR-10.5 Ligand names for unsaturated molecules and groups
Page 25 of 48
DRAFT 2 April 2004
η3-2-methylallyl
26
η4-2-methylidenepropane-
1,3-diido
1,3-diyl
η6-2,3-dimethylidenebutane-
η6-2,3-dimethylidenebutane-
1,4-diido
1,4-diyl
η5-(Z,Z)-pentadienido
η5-(Z,Z)-pentadienyl
η5-cyclopentadienido
η5-cyclopentadienyl
pentamethyl-η5-
pentamethyl-η5-
cyclopentadienido
cyclopentadienyl
tio
n
lR
ec
om
me
n
da
η6-2,2'-biallyl
na
η5-cyclohexadienyl
isi
o
η5-cyclohexadienido
s
η4-2-methylidenepropane-
ov
η7-cycloheptatrienido
η7-tropyl b
η7-cyclooctatrienyl
η7-homotropyl b
PA
C
Pr
η7-cyclooctatrienido
η7-cycloheptatrienyl
1-methyl-η5-borole
IU
B
Me
η5-azacyclopentadienido
η5-azacyclopentadienyl
η5-pyrrolyl
N
DRAFT 2 April 2004
Page 26 of 48
27
η5-phospholyl
s
η5-phosphacyclopentadienido η5-phosphacyclopentadienyl
η5-arsacyclopentadienido
η5-arsacyclopentadienyl
-
om
me
n
η6-borinin-1-uido
B
H
η6-1,4-diborinine-1,4-diuido
ec
2-
H
B
η5-arsolyl
da
As
tio
n
P
B
H
a
η6-boranuidabenzene c
η6-1,4diboranuidabenzene d
isi
o
Cr
bis(η6-benzene)chromium
2.
IU
PA
C
Pr
ov
Examples:
1.
na
lR
The arcs used in these and later examples indicate delocalized charges (by analogy with
the circle in benzene). b If these ligands are regarded as cations they take the ending 'ium'. c
Previously named η6-boratabenzene. d Previously named η6-1,4-diboratabenzene.
Page 27 of 48
DRAFT 2 April 2004
28
tio
n
s
V
3.
ec
U
om
me
n
da
(η7-cycloheptatrienyl)(η5-cyclopentadienyl)vanadium
lR
bis(η8-cyclooctatetraene)uranium
Cr
tris(η3-allyl)chromium
Pr
ov
isi
o
na
4.
5.
IU
PA
C
BMe
Fe
MeB
bis(η6-1-methyl-1-boranuidabenzene)iron
DRAFT 2 April 2004
Page 28 of 48
29
6.
CO
Os
C
tio
n
O
s
PPh3
CO
H PPh3
da
H
7.
O
Ni
C
om
me
n
dicarbonyl(η2-formaldehyde)bis(triphenylphosphane)osmium
PEt3
PEt3
O
ec
(η2-carbon dioxide)bis(triethylphosphane)nickel
lR
8.
NMe2
H
Me
1
na
2
PPh2
Cr
isi
o
OC
CO
CO
ov
tricarbonyl{1-[1-(dimethylamino)ethyl]-2-(diphenylphosphanyl)-η6-benzene}chromium
9.
Pr
Me
Me2Si
PA
C
IU
Page 29 of 48
Br
Nb Br
Br
Me
tribromido[1,1'-(dimethylsilanediyl)bis(2-methyl-η5-cyclopentadienyl)]niobium
10.
DRAFT 2 April 2004
30
Cl
Me2Si
Me2Si
s
Zr
tio
n
Cl
11.
om
me
n
da
dichlorido[(1_3,3a,8a:4a,5_7,7a-η)-4,4,8,8-tetramethyl-1,4,5,8-tetrahydro-4,8-disila-sindacenediyl]zirconium
Cl
Zr
ec
Cl
lR
dichlorido[1,1'-(ethane-1,2-diyl)di(η5-indenyl)]zirconium
isi
o
na
If not all unsaturated atoms of a ligand are involved in bonding, if a ligand can adopt several
bonding modes, or if a ligand bridges several metal atoms, the locants of the ligating atoms
appear in a numerical sequence before the symbol η. Extended coordination over more than
two contiguous carbon atoms should be indicated by, for example, (1_4-η) rather than by
ov
(1,2,3,4-η). The locants and the symbol η are enclosed in parentheses. No superscript on
the symbol η is then necessary.
IU
PA
C
Pr
Examples:
12.
OC
OC
Mo
dicarbonyl[(1_3-η)-cyclohepta-2,4,6-trienyl](η5-cyclopentadienyl)molybdenum
13.
DRAFT 2 April 2004
Page 30 of 48
31
da
tio
n
s
Co
[(1,2,5,6-η)-cyclooctatetraene](η5-cyclopentadienyl)cobalt
om
me
n
14.
CHO
Fe
(CO)3
ec
tricarbonyl[(2_5-η)-(E,E,E)-octa-2,4,6-trienal]iron
isi
o
1
Cr
OC
2
Pr
ov
Examples:
15.
na
lR
Substituents are given the lowest possible numerical locants in the usual manner. However,
the point of attachment to the metal of the first carbon in an allylic system (such as an
'enyl' or a 'dienyl' ligand with an extended chain) has to be numbered in such a way as to
create the longest chain, with the lowest possible locant for the 'yl' suffix.
3
4
IU
PA
C
(η4-buta-1,3-dien-1-yl)carbonyl(η5-cyclopentadienyl)chromium
16.
4
Cr
3
OC
1
Page 31 of 48
2
DRAFT 2 April 2004
32
s
[(1_3-η)-but-2-en-1-yl-4-ylidene]carbonyl(η5-cyclopentadienyl)chromium
tio
n
17.
+
Fe
da
O
CO
om
me
n
CO
OC
tricarbonyl[6-oxo-κO-(2_4-η)-hept-3-en-2-yl]iron(1+)
ec
As indicated in the previous example, the η symbol can, if necessary, be combined with the
κ symbol (see Section IR-10.2.3.3). The symbol η then precedes the ligand name while the
κ symbol is either placed at the end of the ligand name or, for more complicated structures,
lR
after that portion of the ligand name which denotes the particular function in which the
ligating atom is found.
isi
o
na
Examples:
18.
Si
Ti
NBu
Me
t
Cl
ov
Cl
Me
Pr
{[(η5-cyclopentadienyl)dimethylsilyl]-tert-butylaminido-κN}dichloridotitanium
IU
PA
C
19.
PEt3
Cl
4
3
Rh
Et3P
2
O
1
[(E)-η2-but-2-enal-κO]chloridobis(triethylphosphane)rhodium
Use of the symbol η1 for a ligand coordinating via one carbon atom is not generally
recommended for nomenclature purposes. A cyclopentadienyl ligand bonded by only one
DRAFT 2 April 2004
Page 32 of 48
33
s
σ-bond is frequently called σ-cyclopentadienyl or η1-cyclopentadienyl, but cyclopenta2,4-dien-1-yl or cyclopenta-2,4-dien-1-yl-κC1 is more appropriate.
tio
n
Example:
20.
da
H
Fe
om
me
n
OC
OC
dicarbonyl(η5-cyclopentadienyl)(cyclopenta-2,4-dien-1-yl)iron
If an unsaturated hydrocarbon serves as a bridging ligand, the prefix µ (see Sections IR10.2.3.1 and IR-10.2.3.4) is combined with both η and κ, where necessary. The colon is
lR
ec
used to separate the locants of the bridging ligand which indicate binding to different metal
atoms. The priority numbers (as determined from Table VI) of the metal atoms in
polynuclear compounds are placed before the η and κ symbols, which for η are then
enclosed in parentheses, where necessary, as in 1(2_4-η).
isi
o
na
Examples:
21.
Me
C
C
Ni
Pr
ov
Ni
Me
IU
PA
C
(µ-but-2-yne)bis[(η5-cyclopentadienyl)nickel](Ni—Ni)
22.
(CO)3Fe
Fe(CO)3
[trans-µ-(1_4-η:5_8-η)-cyclooctatetraene]bis(tricarbonyliron)
23.
Page 33 of 48
DRAFT 2 April 2004
34
8
7
1
2
tio
n
3
5
4
1
OC
s
6
2
Fe
Fe
OC
CO
CO
da
CO
om
me
n
{µ-[2(1_3,3a,8a-η):1(4_6-η)]azulene}(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe)
24.
1
H
W
2
H
lR
ec
W
(µ-1η5-cyclopentadienediyl-2κC)(µ-2η5-cyclopentadienediyl-1κC)bis[(η5-
na
cyclopentadienyl)hydridotungsten]
O
C
2
Nb
(CO)2
1
Nb
(CO)2
3
Nb
(CO)2
Pr
ov
isi
o
25.
IU
PA
C
µ3-carbonyl-1κC:2κC,O:3κC,O-triangulo-tris[dicarbonyl(η5-cyclopentadienyl)niobium](3 Nb—Nb)
or µ3-2-η2:3-η2-carbonyl-1κC-triangulo-tris[dicarbonyl(η5-cyclopentadienyl)niobium](3 Nb—Nb)
26.
1
2
Cr
Cr
C
O
DRAFT 2 April 2004
Page 34 of 48
35
s
(µ-2-η4-buta-1,3-dienediyl-1κC1,C4)(µ-carbonyl)bis[(η5-cyclopentadienyl)chromium](Cr—Cr)
tio
n
The η-nomenclature can also be extended to π-coordinated ligands containing no carbon
atoms, such as the borazines and pentaphospholyl.
Me
Me
N
om
me
n
B
Me
da
Examples:
27.
N
B
B
Me
N
Me
Me
Cr
CO
OC
CO
lR
ec
tricarbonyl(η6-hexamethyl-1,3,5,2,4,6-triazatriborinane)chromium,
or tricarbonyl(η6-hexamethylcyclotriborazane)chromium
28.
na
P
P
isi
o
P
P
Fe
Me
Me
Me
Me
ov
P
Me
Pr
(pentamethyl-η5-cyclopentadienyl)(η5-pentaphospholyl)iron
PA
C
This nomenclature may also be used for ligands in which σ-bonds are coordinated in a sideon fashion, such as the H−H bond in complexes of dihydrogen (i.e. η2-H2)6 or the
saturated C−H bonds in 'agostic' interactions.7 The η symbol and locants for agostic
IU
interactions are placed separately from other locants at the end of the ligand name. In
Example 30 the agostic bond is denoted by a half arrow.
Page 35 of 48
Examples:
29.
DRAFT 2 April 2004
36
PPri3
OC
CO
s
W
H
tio
n
CO
H
PPri3
da
trans-tricarbonyl(η2-dihydrogen)bis(triisopropylphosphane)tungsten
om
me
n
30.
+
Co
H
H
H 2C
H
H
ec
H
lR
[(1_3-η)-but-2-enyl-η2-C4,H4](η5-cyclopentadienyl)cobalt(1+)
ov
Example:
31.
isi
o
na
For zwitterionic complexes, in which a non-coordinated atom of the ligand carries a charge
which is offset by the opposite charge at the metal atom, the charge on the ligand is
indicated by the appropriate ligand name ending, while the charge of the central atom is not
indicated.
Pr
BPh3
PA
C
Rh
IU
(η4-cycloocta-1,5-diene)(η6-phenyltriphenylborate)rhodium, or
IR-10.2.6
(η4-cycloocta-1,5-diene)(η6-phenyltriphenylboranuide)rhodium
Metallocene nomenclature
DRAFT 2 April 2004
Page 36 of 48
37
s
The first transition metal compound containing only carbocyclic rings as ligands was
bis(cyclopentadienyl)iron, [Fe(η5-C5H5)2], which has a 'sandwich' structure with two
parallel η5- or π-bonded rings. The recognition that this compound was amenable to
da
tio
n
electrophilic substitution, similar to the aromatic behaviour of benzene, led to the
suggestion of the non-systematic name 'ferrocene' and to similar names for other
'metallocenes'.
Examples:
1.
[V(η5-C5H5)2]
2.
[Cr(η5-C5H5)2] chromocene
3.
[Co(η5-C5H5)2] cobaltocene
4.
[Ni(η5-C5H5)2] nickelocene
5.
[Ru(η5-C5H5)2] ruthenocene
6.
[Os(η5-C5H5)2] osmocene
lR
ec
om
me
n
vanadocene
Li
Fe
lithioferrocene or ferrocenyllithium
8.
IU
PA
C
Pr
ov
Examples:
7.
isi
o
na
Metallocene derivatives may be named either by the standard organic suffix (functional)
nomenclature or by prefix nomenclature. The organic functional suffix system is described
in Section P-33 of Ref. 3. For metallocene derivatives the substituent group suffixes
'ocenyl', 'ocenediyl', 'ocenetriyl', etc., are used.
Page 37 of 48
DRAFT 2 April 2004
38
H
s
NMe2
Me
da
tio
n
Fe
9.
om
me
n
1-[1-(dimethylamino)ethyl]ferrocene, or
1-ferrocen-1-yl-N,N-dimethylethan-1-amine
[Ru(η5-C5Me5)2]
decamethylruthenocene, or
bis(pentamethyl-η5-cyclopentadienyl)ruthenium
lR
ec
Substituents on the equivalent cyclopentadienyl rings of the metallocene entity are given
the lowest possible numerical locants in the usual manner. The first ring is numbered 1−5
and the second ring 1'−5'.
COMe
Os
COMe
IU
PA
C
Pr
ov
isi
o
na
Examples:
10.
1,1'-diacetylosmocene, or
osmocene-1,1′-diylbis(ethan-1-one)
11.
Fe
CH2CO2H
1,1'-(4-carboxybutane-1,3-diyl)ferrocene, or
DRAFT 2 April 2004
Page 38 of 48
39
3,5-(ferrocene-1,1′-diyl)pentanoic acid
s
[Cr(η5-C5Me4Et)2]
tio
n
12.
13.
da
1,1'-diethyloctamethylchromocene, or
bis(1-ethyl-2,3,4,5-tetramethyl-η5-cyclopentadienyl)chromium
[Co(η5-C5H4PPh2)2]
om
me
n
1,1'-bis(diphenylphosphanyl)cobaltocene, or
(cobaltocene-1,1'-diyl)bis(diphenylphosphane)
Metallocene nomenclature does not, however, apply to all transition metals. For example,
there are at least two isomers with the empirical formula C10H10Ti but neither has the
ec
regular sandwich structure analogous to that of ferrocene, and so none of these should be
named 'titanocene'. Similarly, 'manganocene' is a misnomer since [Mn(η5-C5H5)2] has a
na
lR
chain structure in the solid state, with no individual sandwich entities, although
decamethylmanganocene, [Mn(η5-C5Me5)2], has a normal sandwich structure, as does
decamethylrhenocene, [Re(η5-C5Me5)2]. With the heavier metals, the occurrence of the
classic ferrocene-type bis(η5-cyclopentadienyl) sandwich structure becomes rare.
isi
o
The name-ending 'ocene' should therefore be confined to discrete molecules of the form
bis(η5-cyclopentadienyl)metal
(and
ring-substituted
analogues),
where
the
ov
cyclopentadienyl rings are essentially parallel, and the metal is in the d-block [i.e. the
terminology does not apply to compounds of the s- or p-block elements such as
Ba(C5H5)2 or Sn(C5H5)2].
PA
C
Pr
The oxidized species may be referred to as metallocenium(n+) salts, although it should be
noted that in this case the ending 'ium' does not carry the usual meaning it has in
substitutive nomenclature, i.e. the addition of a proton to a neutral parent compound. To
avoid this ambiguity, the alternative bis(η5-cyclopentadienyl)iron(1+), for example, is
preferred to ferrocenium(1+) for [Fe(η5-C5H5)2]+. Substituted derivatives are named in a
IU
similar manner, as described before.
Examples:
14.
[Co(η5-C5H5)2][BF4]
bis(η5-cyclopentadienyl)cobalt hexafluorophosphate, or
cobaltocenium hexafluorophosphate
Page 39 of 48
DRAFT 2 April 2004
40
15.
[Co(η5-C5H5)(η5-C5H4COMe)][BF4]
s
(acetyl-η5-cyclopentadienyl)(η5-cyclopentadienyl)cobalt tetrafluoroborate,
tio
n
or acetylcobaltocenium tetrafluoroborate
The oxidized form of osmocene is dinuclear in the solid state, with a long Os–Os bond, so
should not be named using the 'ocenium' nomenclature. However, [Os(η5-C5Me5)2]+ has a
om
me
n
da
mononuclear sandwich structure and may be described as the decamethylosmocenium(1+)
ion, although bis(pentamethyl-η5-cyclopentadienyl)osmium(1+) cation is preferred.
In strong protic acid media, ferrocene is protonated to [Fe(η5-C5H5)2H]+. To avoid
ambiguities, this should be named by the additive procedure, i.e. bis(η5cyclopentadienyl)hydridoiron(1+).
lR
ec
Transition metal complexes derived from ligands with additional rings fused to the
cyclopentadienyl rings are also known. The names of these complexes are derived from the
retained common or semisystematic names of the hydrocarbon ligands, e.g. inden-1-yl
(C9H7), fluoren-9-yl (C13H9), and azulene (C10H8). Thus, [Fe(η5-C9H7)2] is named
bis(η5-indenyl)iron. To avoid possible ambiguities, the use of fusion nomenclature, such as
'benzoferrocene', is strongly discouraged.
na
Many compounds have ligands in addition to two η5-cyclopentadienyl rings. They are
often referred to as metallocene di(ligand) species, e.g. [Ti(η5-C5H5)2Cl2] is frequently
Pr
ov
isi
o
named 'titanocene dichloride'. This practice is discouraged since metallocene nomenclature
applies only to compounds in which the two rings are parallel. Thus, [Ti(η5-C5H5)2Cl2] is
named dichloridobis(η5-cyclopentadienyl)titanium, and [W(η5-C5H5)2H2], [Ti(η5C5H5)2(CO)2]
and
[Zr(η5-C5H5)2Me2]
should
be
named
bis(η5cyclopentadienyl)dihydridotungsten, dicarbonylbis(η5-cyclopentadienyl)titanium, and
bis(η5-cyclopentadienyl)dimethylzirconium, respectively.
IU
PA
C
The bis(cyclooctatetraene) compound [U(η8-C8H8)2] has sometimes been described as
'uranocene'. Related species are obtained from zirconium, [Zr(η8-C8H8)2], and the
lanthanoids, e.g. [Ce(η8-C8H8)2]−. In such complexes, the carbocyclic rings are parallel and
there are certain similarities to ferrocene in the molecular orbital descriptions of their
bonding. However, some lanthanoids also form metal(II) cyclopentadienyl complexes, such
as [Sm(η5-C5Me5)2]. Extension of the 'ocene' nomenclature to [U(η8-C8H8)2] and similar
compounds can therefore lead to confusion and is strongly discouraged.
DRAFT 2 April 2004
Page 40 of 48
41
s
The cyclooctatetraene ring can also function as an η4-ligand, as in [Ti(η4-C8H8)(η8C8H8)]. Compounds of cyclooctatetraene should therefore be named using standard
organometallic nomenclature, e.g. bis(η8-cyclooctatetraene)uranium or [(1_4-η)-
tio
n
cyclooctatetraene](η8-cyclooctatetraene)titanium. The ligand C8H82− is occasionally
da
referred to as 'cyclooctatetraenyl'. This name is incorrect as it can only be used for the (as
yet hypothetical) ligand C8H7.
NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS OF THE MAIN GROUP
ELEMENTS
IR-10.3.1
Introduction
om
me
n
IR-10.3
lR
ec
The nomenclature of organometallic compounds of the main group elements is an area of
current and ongoing development. This section briefly describes key aspects of the naming of
such compounds, leaving a full treatment of the subject to a future publication. Detailed
information on the nomenclature of organic compounds containing the elements of groups
13-16 may be found in Sections P-68 and P-69 of Ref. 3.
PA
C
Pr
ov
isi
o
na
In principle, all organometallic compounds, whether of the transition or main group elements,
can be given names based on the additive system of nomenclature that is applied to
coordination compounds. However, in addition to compounds of the metals and semi-metals,
compounds of elements such as boron, silicon, arsenic and selenium are often considered to
be organometallic, and compounds of these elements are commonly named by notionally
substituting the hydrogen atoms of the parent hydride with the appropriate substituent
groups. Thus, it is recommended that organometallic compounds derived from the elements
of groups 13-16 be named by a substitutive process, while those derived from the elements of
groups 1 and 2 be named using the additive system of nomenclature. Where an
organometallic compound contains two or more central atoms (which may be associated with
different nomenclature systems), a choice must be made to provide the basis of the name. A
general rule is recommended in Section IR-10.3.4.
IU
IR-10.3.2
Page 41 of 48
Organometallic compounds of groups 1 and 2
Organometallic compounds of the elements of groups 1 and 2 are named according to the
additive system of nomenclature, the general definitions and rules of which are given in
Sections IR-9.1 and IR-9.2. Thus, prefixes denoting the organic groups and any other
ligands are placed in alphabetical order before the name of the metal. These prefixes may
adopt either the additive ‘ido’, diido’, etc. endings or the substitutive ‘yl’, ‘diyl’, etc.
endings, the latter practice allowing names in common usage for organic groups to be
DRAFT 2 April 2004
42
tio
n
s
applied unchanged (see Section IR-10.2.2). In the examples below only one of these
alternatives is illustrated, the other form, however, being equally acceptable. The presence
of a hydrogen atom attached to the metal centre must always be indicated (by the prefix
'hydrido') and the name of a cyclic compound may be formed using appropriate locants of a
divalent 'diido' or 'diyl' group to indicate chelate-type bonding to the metal.
(LiMe)n
methyllithium
lR
Examples:
1.
ec
om
me
n
da
Although many organometallic compounds of groups 1 and 2 exist in associated molecular
form (as aggregates) or contain structural solvent, or both, their names are generally based on
the stoichiometric compositions of the compounds. Any solvent is ignored unless it is
specifically desired to draw attention to the extent of aggregation or the nature of any
structural solvent, or both (see Example 3 below). Where an anionic ligand is involved and
little or no structural information is available or is required to be conveyed, a compositional
name (see Chapter IR-5) may be used (see Example 7 below). Note that metallocene
terminology (Section IR-10.2.6) is not recommended for bis(cyclopentadienyl) compounds
of the main group metals (see Examples 8 and 9 below).
BeEtH
ethanidohydridoberyllium
3.
[{Li(OEt2)(µ3-Ph)}4]
tetrakis[(µ3-phenyl)(diethyl ether)lithium],
or tetrakis[(µ3-benzenido)(ethoxyethane)lithium]
4.
Na−CH2=CH
ov
isi
o
na
2.
Pr
ethenidosodium,
or vinylsodium
IU
PA
C
5.
Ph2C(Na)−C(Na)Ph2
1,1,2,2-tetraphenylethane-1,2-diyldisodium
6.
Mg
4
1
3
2
CH2CH2CH=CMe2
2-(4-methylpent-3-en-1-yl)but-2-ene-1,4-diylmagnesium
DRAFT 2 April 2004
Page 42 of 48
43
7.
[{MgI(Me)}n]
tio
n
[Mg(η5-C5H5)2]
bis(η5-cyclopentadienyl)magnesium
9.
[PPh4][Li(η5-C5H5)2]
tetraphenylphosphanium bis(η5-cyclopentadienyl)lithate
da
8.
om
me
n
IR-10.3.3
s
iodido(methanido)magnesium,
or methylmagnesium iodide
Organometallic compounds of groups 13-16
na
lR
ec
Organometallic compounds of the elements of groups 13-16 are named according to the
substitutive system of nomenclature, which is dealt with in Chapter IR-6. Thus, the name of
the parent hydride (formed in accordance with the rules of Section IR-6.2) is modified by a
prefix for each substituent, which is notionally considered to be substituting a hydrogen atom
of the parent hydride. The prefix should be in appropriate substituent form (methyl, chloro,
etc.) and not in ligand form (methanido, chlorido, etc.). Where there is more than one kind of
substituent, the prefixes are cited in alphabetical order before the name of the parent hydride,
parentheses being used to avoid ambiguity, and multiplicative prefixes being used as
necessary. Non-standard bonding numbers are indicated using the λ-convention (see Section
Examples:
AlH2Me
methylalumane
2.
AlEt3
triethylalumane
3.
Me2CHCH2CH2−Al(H)−CH2CH2CHMe2
IU
PA
C
Pr
ov
1.
isi
o
IR-6.2.2.2). An overview of the rules for naming substituted derivatives of parent hydrides is
given in Section IR-6.3, while a detailed exposition may be found in Ref. 3.
Page 43 of 48
bis(3-methylbutyl)alumane
4.
Sb(CH=CH2)3
triethenylstibane (or trivinylstibane)
5.
SbMe5
pentamethyl-λ5-stibane
6.
PhSb=SbPh
diphenyldistibene
7.
GeCl2Me2
dichlorodimethylgermane
8.
GeH(SMe)3
tris(methylsulfanyl)germane
9.
BiI2Ph
diiodo(phenyl)bismuthane
DRAFT 2 April 2004
44
Et3Pb−PbEt3
hexaethyldiplumbane
11.
SnMe2
dimethyl-λ2-stannane
12.
SnBrH2−SnCl2−SnH2Pr
tio
n
s
10.
1-bromo-2,2-dichloro-3-propyltristannane
13.
Me3SnCH2CH2C≡CSnMe3
da
but-1-yne-1,4-diylbis(trimethylstannane)
(EtO)3GeCH2CH2COOMe
ec
Examples:
14.
om
me
n
In the presence of a characteristic group that may be expressed as a suffix (e.g. an amine, a
carboxylic acid, an alcohol, etc.), the organometallic component is prefixed to the
functionalized parent compound as a substituent group as described in Section IR-6.3.1, i.e.
with the parent hydride name ending 'ane' changed to 'anyl' (or 'yl' for the group 14 elements),
'anediyl', etc.
methyl 3-(triethoxygermyl)propanoate
H2As(CH2)4SO2Cl
lR
15.
4-arsanylbutane-1-sulfonyl chloride
OCHCH2CH2GeMe2GeMe2CH2CH2CHO
na
16.
isi
o
3,3'-(1,1,2,2-tetramethyldigermane-1,2-diyl)dipropanal
Pr
ov
Sometimes it may be necessary or preferable to consider a parent hydride in which several
(four or more) skeletal carbon atoms of a hydrocarbon have been replaced by main group
elements. In this method of skeletal replacement the heteroatoms are designated by the 'a'
terms of replacement nomenclature (Table X) cited in the order given by Table VI and
preceded by the appropriate locant(s). The rules for locant numbering are specified in Section
IR-6.2.4.1 and this nomenclature is fully described in Section P-22.2.3.2 of Ref. 3.
IU
PA
C
Examples:
17.
MeSiH2CH2CH2SiH2CH2CH2SiH2CH2CH2SiH2Me
2,5,8,11-tetrasiladodecane
18.
MeSiH2−OPH−OCH2Me
3,5-dioxa-4-phospha-2-silaheptane
19.
HSCH=NOCH2SeCH2ONHMe
3,7-dioxa-5-selena-2,8-diazanon-1-ene-1-thiol
DRAFT 2 April 2004
Page 44 of 48
45
20.
7
Se
1
Se
P
Se
2
4
3
5
da
2,5,7-triselena-1,4-diphosphabicyclo[2.2.1]heptane
tio
n
6
s
P
om
me
n
Cyclic derivatives that have at least four elements from groups 13-16 replacing carbon atoms
in their structures may be named using the 'a' terms of replacement nomenclature (to denote
the heteroatoms) in conjunction with the extended Hantzsch-Widman procedures (to define
the size of the ring). This nomenclature is fully described in Section IR-6.2.4.3 and in
Section P-22.2.2 of Ref. 3 and will not be elaborated further here.
IR-10.3.4
lR
ec
Section P-68 of Ref. 3 offers a more rigorous treatment of the nomenclature of organic
compounds containing the elements of groups 13-16 than can be provided here, and is
illustrated with many further examples.
Priority order for central atoms in organometallic compounds
Central atoms from groups 1-12 only
Pr
IR-10.3.4.1
ov
isi
o
na
When an organometallic compound contains two or more different metal atoms, a choice
must be made to provide the basis of the name. It is thus convenient to classify the possible
central atoms as belonging to either (i) the elements of groups 1-12 (whose compounds are
named according to the additive system of nomenclature) or (ii) the elements of groups 13-16
(whose compounds are named according to the substitutive system).
PA
C
If both or all potential central atoms belong to class (i), then the compound is named
additively using the methodology described in Section IR-9.2.5 and the element priority
sequence given in Table VI (see Section IR-2.15.4). The metal atom last encountered in the
direction of the arrow in Table VI is numbered '1' and listed in the name first; examples are
given in Sections IR-10.2.3.4 and IR-10.2.3.5.
IU
IR-10.3.4.2
Page 45 of 48
Central atoms from both groups 1-12 and groups 13-16
If one possible central atom belongs to class (i) and another to class (ii), then the compound
is named additively using the metal atom of class (i) as the central atom for the basis of the
name. The other atom is named as a prefixed substituent group, i.e. with the parent hydride
DRAFT 2 April 2004
46
tio
n
s
name ending 'ane' changed to 'anyl' (or 'yl' for the group 14 elements), 'anediyl', etc. More
generally, no matter how many metal or semimetal atoms of both classes a compound
contains, the compound is named additively using the metals of class (i), given in element
priority sequence (Table VI), as the basis of the name.
Examples:
1.
om
me
n
da
Often the element of class (ii) will be a donor atom or otherwise a constituent atom of a
ligand coordinating to a class (i) metal, in which case the class (i) element would in any case
be selected as the central atom in the application of the additive nomenclature of coordination
and organotransition metal compounds. Many such examples will be found in earlier parts of
this Chapter.
Li(GePh3)
(triphenylgermyl)lithium
2.
(Me3Si)3CMgC(SiMe3)3
[Mo(CO)5(Sn{CH(SiMe3)2}2)]
{bis[bis(trimethylsilyl)methyl]-λ2-stannyl}pentacarbonylmolybdenum
lR
3.
ec
bis[tris(trimethylsilyl)methyl]magnesium
na
4.
4
1
HgPh
isi
o
Ph 2Sb
ov
[4-(diphenylstibanyl)phenyl](phenyl)mercury
IU
PA
C
Pr
5.
IR-10.3.4.3
Ph
OC
OC
Sb
Mn
Mn
CO
CO
(phenylstibanediyl)bis[dicarbonyl(η5-cyclopentadienyl)manganese]
Central atoms from groups 13-16 only
DRAFT 2 April 2004
Page 46 of 48
47
tio
n
s
If the possible central atoms are both or all from class (ii), then the compound is named
substitutively as described in Section IR-10.3.3 (and in more detail in Section IR-6.3). The
order of priority used to select the parent hydride is based on the seniority order of classes
described in Section P-41 of Ref. 3:
da
N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl > S > Se > Te > C
Thus, for a compound containing both arsenic and lead, the parent hydride would be selected
as AsH3, which has priority over PbH4, the lead atom then appearing in the name as a
Examples:
1.
As(PbEt3)3
tris(triethylplumbyl)arsane
4
1
AsH2
lR
H2Sb
ec
2.
om
me
n
prefixed substituent, often with its own substituent groups.
na
4-(stibanylphenyl)arsane
OMe
SiMe2
1
2
GeMe3
Pr
ov
isi
o
3.
IU
PA
C
[2-(trimethylgermyl)phenyl]methoxydimethylsilane
IR-10.4
Page 47 of 48
4.
Et3PbCH2CH2CH2BiPh2
diphenyl[3-(triethylplumbyl)propyl]bismuthane
5.
SiClH2–Sn(Me)=Sn(Me)–SiClH2
Si,Si′-(1,2-dimethyldistannene-1,2-diyl)bis(chlorosilane)
REFERENCES
DRAFT 2 April 2004
48
s
IU
PA
C
Pr
ov
isi
o
na
lR
ec
5.
6.
7.
tio
n
3.
4.
da
2.
Nomenclature of Inorganic Chemistry, Recommendations 1990, Blackwell Scientific
Publications, Oxford, 1990.
Nomenclature of Organometallic Compounds of the Transition Elements, Pure Appl.
Chem., 71, 1557 (1999).
New Blue Book
Nomenclature of Fused and Bridged Fused Ring Systems, Pure Appl. Chem., 70, 143
(1998).
F.A. Cotton, J. Am. Chem. Soc., 90, 6230 (1968).
D.J. Heinekey and W.J. Oldham, Jr., Chem. Rev., 93, 913 (1993).
M. Brookhart, M.L.H. Green and L.-L. Wong, Prog. Inorg. Chem., 36, 1 (1988).
om
me
n
1.
DRAFT 2 April 2004
Page 48 of 48
IR-11
Solids (Draft March 2004)
tio
n
s
CONTENTS
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
IR-11.1 Introduction
IR-11.1.1 General
IR-11.1.2 Stoichiometric and non-stoichiometric phases
IR-11.2 Names of solid phases
IR-11.2.1 General
IR-11.2.2 Mineral names
IR-11.3 Chemical composition
IR-11.3.1 Approximate formulae
IR-11.3.2 Phases with variable composition
IR-11.4 Point defect (Kröger-Vink) notation
IR-11.4.1 General
IR-11.4.2 Indication of site occupation
IR-11.4.3 Indication of crystallographic sites
IR-11.4.4 Indication of charges
IR-11.4.5 Defect clusters and use of quasi-chemical equations
IR-11.5 Phase nomenclature
IR-11.5.1 Introduction
IR-11.5.2 Recommended notation
IR-11.6 Non-stoichiometric phases
IR-11.6.1 Introduction
IR-11.6.2 Modulated structures
IR-11.6.3 Crystallographic shear structures
IR-11.6.4 Unit cell twinning or chemical twinning
IR-11.6.5 Infinitely adaptive structures
IR-11.6.6 Intercalation compounds
IR-11.7 Polymorphism
IR-11.7.1 Introduction
IR-11.7.2 Use of crystal systems
IR-11.8 Amorphous systems and glasses
IR-11.9 Final remarks
IR-11.10 References
IR-11.1
INTRODUCTION
IR-11.1.1
General
Page 1 of 15
DRAFT 2 April 2004
2
Stoichiometric and non-stoichiometric phases
da
IR-11.1.2
tio
n
s
This chapter deals with some aspects of the nomenclature of solids. However, in cases
where detailed structural information is to be conveyed, fully systematic names can be
difficult to construct. An attempt to deal with this problem is described in Ref. 1.
om
me
n
In binary and multi-component systems, intermediate crystalline phases (stable or
metastable) may occur. Thermodynamically, the composition of any such phase is
variable. In some cases, such as sodium chloride, the possible variation in composition is
very small. Such phases are called stoichiometric. However, in other phases appreciable
variations in composition can occur, for example in wustite (nominally FeO).
lR
ec
These are called non-stoichiometric phases. In general, it is possible to define an ideal
composition relative to which the variations occur. This composition, called the
stoichiometric composition, is usually that in which the ratio of the numbers of different
atoms corresponds to the ratio of the numbers of normal crystallographic sites of different
types in the ideal (ordered) crystal.
isi
o
na
This concept can be used even when the stoichiometric composition is not included in the
homogeneity range of the phase. The term 'non-stoichiometric' does not mean phases with
complex formulae, but those with variable composition (for which the term solid solution
is also used).
NAMES OF SOLID PHASES
IR-11.2.1
General
ov
IR-11.2
Pr
Names for stoichiometric phases, such as NaCl, are derived simply as in Chapter IR-5,
whereas formulae are derived as presented in Chapter IR-4. Although NaCl in the solid
state consists of an infinite network of units, (NaCl)∞, the compound is named sodium
IU
PA
C
chloride and represented symbolically as NaCl.
However, for non-stoichiometric phases and solid solutions, formulae are preferable to
names, since strictly systematic names tend to be inconveniently cumbersome. They
should be used only when unavoidable (e.g. for indexing) and should be constructed in the
following style.
Examples:
DRAFT 2 April 2004
Page 2 of 15
3
Mineral names
tio
n
IR-11.2.2
iron(II) sulfide (iron deficient)
molybdenum dicarbide (carbon excess)
s
1.
2.
om
me
n
da
Mineralogical names should be used only to designate actual minerals and not to define
chemical composition. Thus, the name calcite refers to a particular mineral (contrasted
with
other minerals of similar composition) and is not a term for the chemical compound the
composition of which is properly expressed by the name calcium carbonate.
NiFe2O4 (spinel type)
BaTiO3 (perovskite type)
na
Examples:
1.
2.
lR
ec
A mineral name may, however, be used to indicate the structure type. Where possible, a
name that refers to a general group should replace a more specific name. For example,
large numbers of minerals have been named that are all spinels, but which have widely
differing atomic constituents. In this case, the generic name spinel type should be used
rather than the more specific names chromite, magnetite, etc. The mineral name, printed in
italics, should be accompanied by a representative chemical formula. This is particularly
important for zeolite types.2
CHEMICAL COMPOSITION
IR-11.3.1
Approximate formulae
ov
isi
o
IR-11.3
PA
C
Pr
The formula used in any given case depends upon how much information is to be
conveyed. A general notation, which can be used even when the mechanism of the
variation in composition is unknown, is to put the sign ~ (read as circa, or 'approximately')
before the formula.
IU
Examples:
1.
2.
IR-11.3.2
Page 3 of 15
~ FeS
~ CuZn
If it is desirable to give more information, one of the notations described below may be
used.
Phases with variable composition
DRAFT 2 April 2004
4
2.
(Cu,Ni) denotes the complete range of compositions from pure Cu to pure
Ni.
K(Br,Cl) comprises the range from pure KBr to pure KCl.
om
me
n
Examples:
1.
da
tio
n
s
For a phase where the variation in composition is caused solely or partially by isovalent
substitution, the symbols of the atoms or groups that replace each other may be separated
by a comma and placed together between parentheses. If possible, the formula is written
so that the limits of the homogeneity range, when one or the other of the two atoms or
groups is lacking, are represented. The order of citation should be alphabetical although
exceptions are allowed when it is desired to emphasize structural information.
Phases for which substitution also results in vacant positions are denoted in the same way.
Examples:
3.
na
lR
ec
(Li2,Mg)Cl2 denotes the solid solution intermediate in composition
between
LiCl and MgCl2
4.
(Al2,Mg3)Al6O12 represents the solid solution intermediate in composition
between MgAl2O4( = Mg3Al6O12) and Al2O3 (spinel type) ( =
Al2Al6O12).
isi
o
In general, however, a notation in which there are variables which define composition
should be used. The ranges of the variables can also be indicated. Thus, a phase involving
substitution of atom A for B is written Am+xBn-xCp (0 ≤ x ≤ n). The commas and
ov
parentheses called for above are not then required.
CuxNi1-x (0 ≤ x ≤ 1) is equivalent to (Cu,Ni) but conveys more
Pr
Examples:
5.
information.
KBrxCl1-x (0 ≤ x ≤1) is equivalent to K(Br,Cl).
7.
Li2-2xMgxCl2 (0 ≤ x ≤1) is equivalent to (Li2,Mg)Cl2 but shows explicitly
IU
PA
C
6.
8.
that one vacant cation position appears for every 2 Li+ replaced by Mg2+.
Co1-xO indicates that there are vacant cation sites; for x = 0 the formula
corresponds to the stoichiometric composition CoO.
DRAFT 2 April 2004
Page 4 of 15
5
9.
CaxZr1-xO2-x indicates that Zr has been partly replaced by Ca, resulting in
tio
n
s
vacant anion sites; for x = 0 the formula corresponds to the stoichiometric
composition ZrO2.
Examples:
10.
11.
12.
13.
om
me
n
da
If the variable x is limited this may be shown by using δ or ε instead of x. A specific
composition or composition range can be indicated by stating the actual value of the
variable x (or δ, or ε). This value can be written in parentheses after the general formula.
However, the value of the variable may also be introduced in the formula itself. This
notation can be used both for substitutional and for interstitial solid solutions.2
Fe3xLi4-xTi2(1-x)O6 (x = 0.35), or Fe1.05Li3.65Ti1.30O6
LaNi5Hx (0 < x < 6.7)
Al4Th8H15.4
Ni1-δO
POINT DEFECT (KRÖGER-VINK) NOTATION
IR-11.4.1
General
lR
ec
IR-11.4
IR-11.4.2
isi
o
na
As well as the chemical composition, information about point defects, site symmetry, and
site occupancy can be given by using additional symbols. These symbols may also be used
to write quasi-chemical equilibria between point defects.3
Indication of site occupancy
Pr
ov
In a formula, the main symbols indicate the species present at a certain site, defined with
respect to empty space. This will generally be the symbol of an element. If a site is vacant
this is denoted by the italicized symbol V. (In certain contexts other symbols, such as a
square box, , are used for vacancies, but the use of italicized V is preferred, the element
IU
PA
C
vanadium being written with the upright symbol V).
The site and its occupancy in a structure of ideal composition are represented by right
lower indexes. The first index indicates the type of site, and the second index (if used),
separated from the first by a comma, indicates the number of atoms on this site. Thus, an
atom A on a site normally occupied by A in the ideal structure is expressed by AA; an
atom A on a site normally occupied by B is expressed AB; and MM,1-xNM,xMN,xNN,1-x
stands for a disordered alloy, where the ideal composition is MMNN with all M atoms on
one type of crystallographic site and all N atoms on a second type of crystallographic site.
Page 5 of 15
DRAFT 2 April 2004
6
An alternative description is (M1-xNx)M(MxN1-x)N. A species occupying an interstitial site
MgMg,2-xSnMg,xMgSn,xSnSn,1-x shows that in Mg2Sn some of the Mg
da
atoms are present on Sn sites and vice versa.
2.
tio
n
Examples:
1.
s
(i.e. a site which is unoccupied in the ideal structure) is indicated by the subscript 'i'.
(Bi2-xTex)Bi(BixTe3-x)Te shows that in Bi2Te3 some of the Bi atoms are
om
me
n
present on Te sites and vice versa.
3.
NaNa,1-xVNa,xClCl,1-xVCl,x shows that x Na and x Cl sites in NaCl are
vacant, giving Schottky defects.
4.
CaCa,1FF,2-xVF,xFi,x shows that in CaF2, x F sites are vacant, while x F
ions are situated on interstitial sites, creating Frenkel defects.
(Ca0.15Zr0.85)Zr(O1.85V0.15)O, or CaZr,0.15ZrZr,0.85OO,1.85VO,0.15 shows
ec
5.
that in CaO-stabilized ZrO2, 0.85 of the Zr sites are occupied by Zr, 0.15 of
lR
the Zr sites are occupied by Ca, and that, of the two oxygen sites,
are occupied by oxygen ions, leaving 0.15 sites vacant.
na
1.85 sites
6.
VV,1CC,0.8VC,0.2 shows that 0.2 C-sites are vacant in a vanadium carbide.
NaNa → VNa + Na(g) indicates the evaporation of a Na atom, leaving
a sodium vacancy in the lattice.
ov
Examples:
7.
isi
o
The defect symbols can be used in writing quasi-chemical reactions.
Pr
behind
8.
1/2Cl2(g) + VCl → ClCl indicates the incorporation of a chlorine atom, from
PA
C
a chlorine molecule, on a vacant chlorine site in the lattice.
IU
IR-11.4.3
Indication of crystallographic sites
Crystallographic sites can be distinguished by subscripts, e.g. tet, oct and dod, denoting
tetrahedrally, octahedrally and dodecahedrally coordinated sites, respectively. The use of
subscripts such as a, b, . . ., which are not self-explanatory, is not approved. In some
cases, such as oxides and sulfides, the number of subscripts can be reduced by defining
specific symbols to indicate site symmetries, e.g. ( ) for tetrahedral sites, [ ] for octahedral
DRAFT 2 April 2004
Page 6 of 15
7
2.
Indication of charges
om
me
n
IR-11.4.4
MgtetAloct,2O4 or (Mg)[Al2]O4 denotes a normal spinel.
FetetFeoctNioctO4 or (Fe)[FeNi]O4 denotes NiFe2O4 (inverse spinel type)
da
Examples:
1.
tio
n
s
sites, { } for dodecahedal sites. To avoid confusion, such enclosing marks should be
restricted to cases where they are not being used to express multiplication. The meaning
of the symbols should be clearly stated in the text.
Charges are indicated by a right upper index. When formal charges are given, the usual
convention holds: one unit of positive charge is indicated by a superscript +, n units of
positive charge by a superscript n+, one unit of negative charge by superscript _, n units of
ec
_
negative charge by a superscript n . Thus An+ denotes n units of formal positive charge
on an atom of symbol A. In defect chemistry, charges are defined preferably with respect
to the ideal unperturbed crystal. In this case, they are called effective charges. One unit of
positive effective charge is shown by a superscript dot, •, (not to be confused with the
isi
o
na
lR
radical dot described in Section IR-4.6.2) and one unit of negative effective charge by a
prime '; n units of effective charge are indicated by superscript n• or n'. The use of double
dots •• or double primes " in the case of two effective charges is also allowed. Thus A2•
and A•• indicate that an atom of symbol A has two units of effective positive charge. Sites
that have no effective charge relative to the unperturbed lattice may be indicated explicitly
by a superscript cross, i.e. 'x'.
and
x
•
x
Li Li,1-2x Mg Li,x VLi,x
′ ClCl
are
equivalent
ov
Examples:
•
1.
Li Li,1-2x Mg Li,x VLi,x
′ ClCl
expressions for a substitutional solid solution of MgCl2 in LiCl.
Pr
x
′ O3 and YY,1-2
′ Ox3 are equivalent expressions for
YY,1-2 x Zr •Y,2 x O′i,x
x Z r′Y,2 x O ′i,x
an interstitial solid solution of ZrO2 in Y2O3.
PA
C
2.
3.
•
Ag Ag,1-x V Ag,
′ x Agi,x Cl Cl indicates that a fraction x of the Ag+ ions is removed
IU
from the Ag sites to interstitial sites, leaving the silver site vacant.
Formal charges may be preferred in cases where the unperturbed crystal contains an
element in more than one oxidation state.
Examples:
Page 7 of 15
DRAFT 2 April 2004
8
2+
3+
2La La,1-3x La La,2+2 x VLa, x S4 (0 < x < 1/3)
5.
+
3+
+
2Cu Cu,2 -x Fe Cu,x Tl Tl Se Se,1+2 x Se Se,1-2 x (0 < x < 1/2) shows that Fe3+
s
4.
tio
n
_ _
partly replaces Cu+ in Cu+2Tl+Se2 Se
da
Free electrons are denoted by e', free holes by h•. As crystals are macroscopically neutral
bodies, the sums of the formal charges and of the effective charges must be zero.
interstitial M2+ ion
_
interstitial X ion
M••
i
M2+ ion vacancy
_
X ion vacancy
VM
′′
M xi
VM
′
na
M+ ion vacancy
X atom vacancy
x
VX
Q3+ ion at M2+ site
X xi
M•i
x
VM
normal M2+ ion
_
normal X ion
V •x
interstitial M+ ion
M atom vacancy
ec
interstitial X atom
X ′i
lR
interstitial M atom
om
me
n
_
Table IR-11.1 Examplesa of defect notation in M2+(X )2 containing a foreign ion Q
M xM
X xX
Q•M
Q2+ ion at M2+ site
QxM
Q+ ion at M2+ site
Q′M
free electron
e′
free hole
h•
_
Consider an ionic compound M2+(X )2. The formal charge on M is 2+, the formal
charge on X is 1_. If an atom X is removed, one negative unit of charge remains on the
isi
o
a
vacant X site. The vacancy is neutral with respect to the ideal MX2 lattice and is therefore
ov
x
indicated by V x or V X
. If the electron is also removed from this site, the resultant
vacancy is effectively positive, i.e. V •x . Similarly, removal of an M atom leaves V M ,
PA
C
Pr
removal of an M+ ion leaves V M
′ , removal of an M2+ ion leaves V M
′′ . If an impurity with
a formal charge of three positive units Q3+ is substituted on the M2+ site, its effective
charge is one positive unit. Therefore it is indicated by Q•M .
IU
IR-11.4.5
Defect clusters and use of quasi-chemical equations
Pairs or more complicated clusters of defects can be present in a solid. Such a defect
cluster is indicated between parentheses. The effective charge of the cluster is indicated as
an upper right index.
Examples
DRAFT 2 April 2004
Page 8 of 15
9
(Ca •K V ′K )x denotes a neutral defect pair in a solid solution, for example of
1.
s
CaCl2 in KCl.
•
(V Pb
′′ VCl )′ or (V Pb VCl )′ indicates a charged vacancy pair in PbCl2.
tio
n
2.
′′ → (Cr MgV Mg )′ describes the association reaction of a Cr3+
Cr •Mg + V Mg
om
me
n
Examples:
3.
da
Quasi-chemical reactions may be written for the formation of these defect clusters.
impurity in MgO with magnesium vacancies.
′′ → (Cr MgV MgCr Mg ) gives another possible association
2Cr •Mg + V Mg
x
4.
reaction in the system of Example 3.
PHASE NOMENCLATURE
IR-11.5.1
Introduction
na
lR
IR-11.5
ec
Gd•Ca + F ′i → (GdCa F i )x describes the formation of a dipole between a
Gd3+ impurity and a fluorine interstitial in CaF2.
5.
Recommended notation
Pr
IR-11.5.2
ov
isi
o
The use of the Pearson notation4 (see also Section IR-3.5.3) is recommended for the
designation of the structures of metals and solid solutions in binary and more complex
systems. The use of Greek letters, which do not convey the necessary information, and of
the Strukturbericht designations, which are not self-explanatory, is not approved.
PA
C
The Pearson symbol consists of three parts: first, a lower-case italic letter (a, m, o, t, h, c)
designating the crystal system; second, an italic capital letter (P,S,F,I,R) designating the
lattice setting and, finally, a number designating the number of atoms in the conventional
unit cell. Table IR-3.1 summarizes the system.
IU
Examples:
1.
Page 9 of 15
2.
Cu, symbol (cF4), indicates copper of cubic symmetry, with face-centred
lattice, containing 4 atoms per unit cell.
NaCl, symbol (cF8), indicates a cubic face-centred lattice with 8 atoms per
unit cell.
DRAFT 2 April 2004
10
CuS(hP12), indicates a hexagonal primitive lattice with 12 atoms per unit
3.
tio
n
s
cell.
da
If required, the Pearson symbol can be followed by the space group and a prototype
formula.
Example:
4.
IR-11.6.
NON-STOICHIOMETRIC PHASES
IR-11.6.1
Introduction
om
me
n
Ag1.5CaMg0.5(hP12, P63/mmc) (MgZn2 type).
na
lR
ec
There are a number of special problems of nomenclature for non-stoichiometric phases
which have arisen with the improvements in the precision with which their structures can
been determined. Thus, there are references to homologous series, non-commensurate and
semi-commensurate structures, Vernier structures, crystallographic shear phases, Wadsley
defects, chemical twinned phases, infinitely adaptive phases, and modulated structures.
Many of the phases that fall into these classes have no observable composition ranges
although they have complex structures and formulae; an example is Mo17O47. These
Modulated structures
ov
IR-11.6.2
isi
o
phases, despite their complex formulae, are essentially stoichiometric and possession of a
complex formula must not be taken as an indication of a non-stoichiometric compound (cf.
Section IR-11.1.2).
IU
PA
C
Pr
Modulated structures possess two or more periodicities in the same direction of space. If
the ratio of these periodicities is a rational number, the structures are called
commensurate; if the ratio is irrational, the structures are called non-commensurate or
incommensurate. Commensurately modulated structures exist in many stoichiometric and
non-stoichiometric compounds; they may be regarded as superstructures and be described
by the usual rules. Non-commensurately modulated structures occur in several
stoichiometric compounds (and some elements), usually in a limited temperature range,
e.g. U, SiO2, TaS2, NbSe3, NaNO2, Na2CO3, and Rb2ZnBr4.
Many modulated structures can be regarded as being composed of two or more
substructures. The substructure with the shortest periodicity often represents a simple
basic structure, while the other periodicities cause modulations of the basic structure. The
DRAFT 2 April 2004
Page 10 of 15
11
Examples
1.
da
tio
n
s
basic structure often remains unchanged within a certain composition range, while the
other substructures take up the change in stoichiometry. If this change takes place
continuously, a non-stoichiometric phase with a non-commensurate structure results. If the
change occurs discontinuously, a series of (essentially stoichiometric) homologous
compounds with commensurate structures (superstructures of the basic structure) may
result or, in the intermediate case, a series of compounds with semi-commensurate or
Vernier structures.
om
me
n
MnnSi2n-m
The structure is of the TiSi2 type which has two atom substructures, the Mn array
being identical to that of the Ti array in TiSi2 and the Si2 array being identical to
that of the Si2 array in TiSi2. Removal of Si leads to a composition MnnSi2n-m in
YF2+xO
na
2.
lR
ec
which the Mn array is completely unchanged. The Si atoms are arranged in rows
and, as the Si content falls, the Si atoms in the rows spread out. In this case there
will be a Vernier relationship between the Si atom rows and the static Mn positions
which will change as the composition varies, giving rise to non-commensurate
structures.
isi
o
The structure is of the fluorite type with extra sheets of atoms inserted into the
parent YX2 structure. When these are ordered, a homologous series of phases
ov
results. When they are disordered, there is a non-commensurate, nonstoichiometric phase, while partial ordering will give a Vernier or semicommensurate effect. Other layer structures can be treated in the same way.
PA
C
Pr
Misfit structures consist of two or more different, often mutually non-commensurate, units
which are held together by electrostatic or other forces; no basic structure can be defined.
The composition of compounds with misfit structures is determined by the ratio of the
periodicities of their structural units and by electroneutrality.
IU
Examples:
3.
Page 11 of 15
Sr1-pCr2S4-p with p = 0.29, where chains of compositions Sr3CrS3 and
Sr3-xS lie in tunnels of a framework of composition Cr21S36; the three units
are mutually non-commensurate.
4.
LaCrS3, which is built from non-commensurate sheets of (LaS)+ and
_
(CrS2) .
DRAFT 2 April 2004
12
Crystallographic shear structures
s
IR-11.6.3
om
me
n
da
tio
n
Crystallographic shear planes (CS planes) are planar faults in a crystal that separate two
parts of the crystal which are displaced with respect to each other. The vector describing
the displacement is called the crystallographic shear vector (CS vector). Each CS plane
causes the composition of the crystal to change by a small increment because the sequence
of crystal planes that produces the crystal matrix is changed at the CS plane. (From this it
follows that the CS vector must be at an angle to the CS plane. If it were parallel to the
plane, the succession of crystal planes would not be altered and no composition change
would result. A planar boundary where the displacement vector is parallel to the plane is
more properly called an antiphase boundary).
TinO2n-1
The parent structure is TiO2 (rutile type). The CS planes are the (121)
ov
Examples:
1.
isi
o
na
lR
ec
Because each CS plane changes the composition of the crystal slightly, the overall
composition of a crystal containing a population of CS planes will depend upon the
number of CS planes present and their orientation. If the CS planes are disordered, the
crystals will be non-stoichiometric, the stoichiometric variation being due to the CS plane
'defect'. If the CS planes are ordered into a parallel array, a stoichiometric phase with a
complex formula results. In this case, a change in the separation of the CS planes in the
ordered array will produce a new phase with a new composition. The series of phases
produced by changes in the spacing between CS planes forms an homologous series. The
general formula of a particular series will depend upon the type of CS plane in the array
and the separation between the CS planes. A change in the CS plane may change the
formula of the homologous series.
PA
C
Pr
planes. Ordered arrays of CS planes can exist, producing an homologous
series of oxides with formulae Ti4O7, Ti5O9, Ti6O11, Ti7O13. Ti8O15,
Ti9O17. The series formula is TinO2n-1, with n between 4 and 9.
IU
2.
(Mo,W)nO3n-1
The parent structure is WO3. The CS planes are the (102) planes. Ordered
arrays of CS planes can form, producing oxides with formulae Mo8O23,
Mo9O26, (Mo,W)10O29, (Mo,W)11O32, (Mo,W)12O35, (Mo,W)13O38, and
(Mo,W)14O41. The series formula is (Mo,W)nO3n-1, with n between 8 and
14.
3.
WnO3n-2
DRAFT 2 April 2004
Page 12 of 15
13
The parent structure is WO3. The CS planes are the (103) planes. Ordered
arrays of CS planes can form, producing oxides with formulae WnO3n-2,
tio
n
IR-11.6.4
s
with n between approximately 16 and 25.
Unit cell twinning or chemical twinning
Example:
1.
om
me
n
da
This is a structure-building component in which two constituent parts of the structure are
twin-related across the interface. The twin plane changes the composition of the host
crystal by a definite amount (which may be zero). Ordered, closely spaced arrays of twin
planes will lead to homologous series of phases. Disordered twin planes will lead to nonstoichiometric phases in which the twin planes serve as the defects. There is a close
parallel between chemical twinning and crystallographic shear.
(Bi,Pb)nSn-4
ec
The parent structure is PbS which has the cF8 (NaCl type) structure. The
twin planes are (311) with respect to the PbS unit cell. Two members of the
homologous series are known, Bi8Pb24S36 and Bi8Pb12S24, but other
Infinitely adaptive structures
isi
o
IR-11.6.5
na
lR
members are found in the quaternary Ag-Bi-Pb-S system. The difference
between compounds lies in the separation of the twin planes; each structure
is built from slabs of PbS of varying thickness, alternate slabs being
twinned across (311) with respect to the parent structure.
Pr
ov
In some systems it would appear that any composition can yield a fully ordered crystal
structure over certain temperature and composition ranges. As the composition changes,
so the structure changes to meet this need. The term infinitely adaptive structures has been
applied to this group of substances.5
PA
C
Examples:
1.
IU
2.
IR-11.6.6
Page 13 of 15
Compounds in the Cr2O3-TiO2 system between the composition ranges
(Cr,Ti)O2.93 and (Cr,Ti)O2.90.
Compounds in the Nb2O5-WO3 system with block-type structure between
the composition limits Nb2O5 and 8WO3.9Nb2O5 (Nb18W8O69).
Intercalation compounds
DRAFT 2 April 2004
14
da
tio
n
s
There are several materials in which a guest species is inserted into a host matrix. The
process is called intercalation, and the product is called an intercalation compound.
Common examples of intercalated materials are found in the clay silicates, layered
dichalcogenides, and electrode materials for lithium batteries; graphite intercalation is
considered in detail in Ref. 6. Intercalated materials can be designated by conventional
chemical formulae such as LixTaS2 (0<x<1) or by host-guest designations, such as
TaS2:xLi (0<x<1). If the stoichiometry is definite, ordinary compound designations may
be used, e.g. TaS2(N2H4)4/3, TiSe2(C5H5N)1/2, and KC8. (In this particular instance,
om
me
n
fractional indexes may be used).
Many intercalation compounds are layered structures and intercalation is a twodimensional reaction. The term insertion is sometimes used for three-dimensional
examples, as in the tungsten bronzes, e.g. NaxWO3, and the spinels, e.g. LixMn2O4.
POLYMORPHISM
IR-11.7.1
Introduction
ec
IR-11.7
isi
o
na
lR
A number of chemical compounds and elements change their crystal structure with
external conditions such as temperature and pressure. These various structures are termed
polymorphic forms of the compounds, and in the past have been designated using a
number of labelling systems, including Greek letters and Roman numerals; the use of such
non-systematic labels is discouraged. A rational system based upon crystal structure
should be used wherever possible (cf. Sections IR-3.5.3 and IR-4.2.5).
Pr
Use of crystal systems
Polymorphs are indicated by adding an italicized symbol denoting the crystal system after
the name of formula. The symbols used are given in Table IR-3.1. For example, zinc
sulfide (c) or ZnS(c) corresponds to the zinc blende structure or sphalerite, and ZnS(h) to
the wurtzite structure. Slightly distorted lattices may be indicated by using the circa sign
~. Thus, a slightly distorted cubic lattice would be expressed as (~c). In order to give more
information, simple well-known structures should be designated by giving the type
compound in parentheses whenever possible. For example, AuCd above 343 K should be
designated AuCd (CsCl type) rather than AuCd(c).
IU
PA
C
IR-11.7.2
ov
Polytypes and polytypoids can be regarded as a special form of polymorphism and are
treated in more detail in Ref. 7.
DRAFT 2 April 2004
Page 14 of 15
15
FINAL REMARKS
tio
n
IR-11.8
s
Properties which strongly depend on lattice and point symmetries may require the addition
of the space group to the crystal system abbreviation. For more details see Ref. 8.
REFERENCES
1.
2.
Nomenclature of Inorganic Structure Types, Acta. Cryst., A46, 1 (1990).
Chemical Nomenclature and Formulation of Compositions of Synthetic and Natural
Zeolites, Pure Appl. Chem., 1, 1091 (1979).
F.A. Kröger and H.J. Vink, Solid State Phys., 3, 307 (1956).
W.B. Pearson, A Handbook of Lattice Spacings and Structures of Metals and Alloys,
Vol. 2, Pergamon Press, Oxford, 1967, pp. 1,2. For tabulated lattice parameters and
data on elemental metals and semi-metals, see pp 79-91. See also, P. Villars and
L.D. Calvert, Pearson's Handbook of Crystallographic Data for Intermetallic
Phases, Vols. 1-3, American Society for Metals, Metals Park, Ohio, USA, 1985.
J.S. Anderson. J. Chem. Soc., Dalton Trans., 1107 (1973).
Graphite Intercalation Compounds, Chapter II-6 in Nomenclature of Inorganic
Chemistry II, Recommendations 2000, Royal Society of Chemistry, 2001.
Nomenclature of Polytype Structures, Acta Cryst., A40, 399 (1984). See also, Acta
Cryst., A33, 681 (1977).
Structural Phase Transition Nomenclature, Acta Cryst., A54, 1028 (1998).
IU
PA
C
Pr
ov
8.
na
7.
isi
o
5.
6.
lR
ec
3.
4.
om
me
n
1-11.9
da
This Chapter deals with the basic nomenclature of solid state chemistry. In some areas,
such as amorphous systems and glasses, the nomenclature needs further development. The
reader is also referred to the work of the International Union of Crystallography.
Page 15 of 15
DRAFT 2 April 2004
Names, symbols and atomic numbers of the elements (see also Section IR-3.1)
PA
C
IU
Page 1 of 2
29
96
110
105
66
99
68
63
100
9
87
64
31
neodymium
neon
neptunium
nickel
niobium
nitrogen j
nobelium
osmium
oxygen
palladium
phosphorus
platinum
plutonium
DRAFT 2 April 2004
en
da
tio
ns
Atomic number
Ge
Au e
Hf
Hs
He
Ho
Hf
In
I
Ir
Fe g
Kr
La
Lr
Pb h
Li
Lu
Mg
Mn
Mt
Md
Hg i
Mo
32
79
72
108
2
67
1
49
53
77
26
36
57
103
82
3
71
12
25
109
101
80
42
Nd
Ne
Np
Ni
Nb
N
No
Os
O
Pd
P
Pt
Pu
60
10
93
28
41
7
102
76
8
46
15
78
94
m
germanium
gold
hafnium
hassium
helium
holmium
hydrogen
indium
iodine
iridium
iron
krypton
lanthanum
lawrencium
lead
lithium
lutetium
magnesium
manganese
meitnerium
mendelevium
mercury
molybdenum
m
Cu d
Cm
Ds
Db
Dy
Es
Er
Eu
Fm
F
Fr
Gd
Ga
Symbol
co
copper
curium
darmstadtium
dubnium
dysprosium
einsteinium
erbium
europium
fermium
fluorine
francium
gadolinium
gallium
89
13
95
51
18
33
85
56
97
4
83
107
5
35
48
55
20
98
6
58
17
24
27
Name
Re
Ac
Al
Am
Sb b
Ar
As
At
Ba
Bk
Be
Bi
Bh
B
Br
Cd
Cs
Ca
Cf
C
Ce
Cl
Cr
Co
Pr
o
actinium
aluminium a
americium
antimony
argon
arsenic
astatine
barium
berkelium
beryllium
bismuth
bohrium
boron
bromine
cadmium
caesium c
calcium
californium
carbon
cerium
chlorine
chromium
cobalt
Atomic number
al
Symbol
ion
Name
vis
Table I
Atomic number
en
da
tio
ns
Sr
S
Ta
Tc
Te
Tb
Tl
Th
Tm
Sn o
Ti
Wp
U
V
Xe
Yb
Y
Zn
Zr
m
strontium
sulfur n
tantalum
technetium
tellurium
terbium
thallium
thorium
thulium
tin
titanium
tungsten
uranium
vanadium
xenon
ytterbium
yttrium
zinc
zirconium
m
84
19
59
61
91
88
86
75
45
37
44
104
62
21
106
34
14
47
11
Symbol
co
Po
Kk
Pr
Pm
Pa
Ra
Rn
Re
Rh
Rb
Ru
Rf
Sm
Sc
Sg
Se
Si
Ag l
Na m
Name
Re
polonium
potassium
praseodymium
promethium
protactinium
radium
radon
rhenium
rhodium
rubidium
ruthenium
rutherfordium
samarium
scandium
seaborgium
selenium
silicon
silver
sodium
Atomic number
al
Symbol
ion
Name
38
16
73
43
52
65
81
90
69
50
22
74
92
23
54
70
39
30
40
vis
a The alternative spelling 'aluminum' is commonly used. b The element symbol Sb derives from the name stibium.
c The alternative spelling 'cesium' is commonly used. d The element symbol Cu derives from the name cuprum. e
IU
PA
C
Pr
o
The element symbol Au derives from the name aurum. f The hydrogen isotopes 2H and 3H are named deuterium
and tritium, respectively, for which the symbols D and T may be used. However, 2H and 3H are preferred (see
Section IR-3.3.2). g The element symbol Fe derives from the name ferrum. h The element symbol Pb derives from
the name plumbum. i The element symbol Hg derives from the name hydrargyrum. j The name azote provides the
root 'az' for nitrogen. k The element symbol derives K from the name kalium. l The element symbol Ag derives
from the name argentum. m The element symbol Na derives from the name natrium. n The name theion provides
the root 'thi' for sulfur. o The element symbol Sn derives from the name stannum. p The element symbol W
derives from the name wolfram.
DRAFT 2 April 2004
Page 2 of 2
130
140
150
160
170
180
190
untrinilium
unquadnilium
unpentnilium
unhexnilium
unseptnilium
unoctnilium
unennilium
200
201
202
binilnilium
binilunium
binilbium
Bnn
Bnu
Bnb
trinilnilium
quadnilnilium
pentnilnilium
Tnn
Qnn
Pnn
ennilnilium
Enn
IU
900
Utn
Uqn
Upn
Uhn
Usn
Uon
Uen
al
ion
vis
Pr
o
PA
C
300
400
500
m
Symbol
Uuu
Uub
Uut
Uuq
Uup
Uuh
Uus
Uuo
Uue
Ubn
Ubu
m
Name
unununiumb
ununbium
ununtrium
ununquadium
ununpentium
ununhexium
ununseptium
ununoctium
ununennium
unbinilium
unbiunium
co
Atomic number
111
112
113
114
115
116
117
118
119
120
121
en
da
tio
ns
Temporary names for elements of atomic number greater than
110.a
Re
Table II
a
These names are used only when the permanent name has not yet been assigned by IUPAC
(see Section IR-3.1.1). b One may also write, for example, 'element 111'.
Page 1 of 1
DRAFT 2 April 2004
1
Suffixes and endings a
a
Termination vowel for the prefixes of skeletal replacement and HantzschWidman nomenclature: e.g. oxa, aza.
en
da
tio
ns
Table III
In boron nomenclature: e.g. carba, thia. (Also in natural product
nomenclature, e.g. carbayohimban).
ane
Ending for names of neutral saturated parent hydrides of elements of
Groups 13-17: e.g. phosphane, cyclohexane, cubane.
Hantzsch-Widman termination for names of all saturated n-membered
ings. (See also 'ine').
Combined ending ('ane') and suffix ('ium') describing a cation resulting from
hydron addition to a parent hydride denoted by an 'ane' ending.
ate
General ending for most polyatomic anions: e.g. nitrate, acetate,
hexacyanidoferrate. Some anions are exceptions, having names which end
in 'ite' or 'ide'.
ato
Ending to name of an inorganic or organic ion acting as a ligand: e.g. sulfato,
glycinato.
diyl
Suffix composed of the suffix 'yl' and the multiplying prefix 'di' indicating
the loss of two hydrogen atoms from the same or different atoms leaving a
residue which forms only two single bonds: e.g. phosphanediyl, HP<.
ene
Ending for names of unsaturated acyclic and cyclic hydrocarbons: e.g.
pentene, cyclohexene, benzene, azulene. (See also 'ocene').
ion
al
Re
co
m
m
anium
Ending for names of unsaturated homogeneous and heterogeneous chain
and ring compounds: e.g. triazene.
Ending resulting from the change of the 'ene' ending in cyclic mancude
ring systems to 'eno' to form prefixes in fusion nomenclature.
ic
Ending for many acids, both inorganic and organic: e.g. sulfuric acid, acetic
acid, benzoic acid.
Pr
o
ide
vis
eno
Ending for names of certain monoatomic anions: e.g. chloride, sulfide.
PA
C
Ending for names of the more electronegative constituent in compositional
names: e.g. disulfur dichloride.
In names of homopolyatomic anions: e.g. triiodide.
IU
In some heteropolyatomic anions: e.g. cyanide, hydroxide.
Page 1 of 4
Suffix for anions formed by removal of one or more hydrons from a
parent hydride: e.g. hydrazinide, methanide.
DRAFT 2 April 2004
2
Modification to name of an anion ending in 'ide', acting as a ligand: e.g.
chlorido, disulfido.
ine
Ending for non-systematic names of Group 15 hydrides: e.g. hydrazine
(N2H4) and phosphine (PH3).
en
da
tio
ns
ido
Ending in the Hantzsch-Widman system for unsaturated heteromonocycles
and saturated nitrogeneous heteromonocycles with three-, four-, and fivemembered rings: e.g. 'irine', 'epine', 'iridine', 'etidine', 'olidine'.
Ending for nitrogeneous heterocyclic parent hydride names: e.g. pyridine,
acridine.
Termination for names of anions of certain non-carbon acids having names
ending in 'inic': e.g. phosphinate, sulfinate.
inato
Modification to the termination of the name of an 'inate' anion when acting as
a ligand.
ino
Ending for some prefix names: e.g. sulfino, amino, hydrazino.
inoyl
Combined affix resulting when the ending 'ic acid' is been changed to 'oyl':
e.g. from phosphinic acid to phosphinoyl, from sulfinic acid to sulfinoyl.
io
Non preferred ending for some substituent groups derived from
onium cations: e.g. ammonio, pyridinio.
ite
Ending for anions (esters and salts) of oxoacids having the 'ous' ending in
the acid name: e.g. sulfite.
ito
Termination for the name of an anion derived from an 'ous' acid, and acting
as a ligand: e.g. sulfito.
ium
Ending of names of many elements, and for the name of any new element:
e.g. helium, seaborgium.
vis
ion
al
Re
co
m
m
inate
Ending for many electropositive constituents of compositional names.
Pr
o
Suffix to indicate addition of a hydron to a parent structure: e.g. methanium,
azanium, pyridinium.
Ending for names of cations derived from metallocenes: e.g. ferrocenium.
Ending indicating a negatively charged ligand, usually appearing as 'ido', 'ito',
'ato' e.g. bromido.
PA
C
o
IU
Ending for the names of many inorganic and organic substituent groups: e.g.
amino, chloro, piperidino.
Ending for infixes used to indicate replacement of oxygen atoms and/or
hydroxyl groups: e.g. 'thio', 'amido', 'nitrido'.
DRAFT 2 April 2004
Page 2 of 4
3
Suffix for the names of bis(cyclopentadienyl)metal compounds and their
derivatives: e.g. ferrocene.
onate
Combined affix for the name of an anion derived from an 'onic' acid: e.g.
phosphonic acid gives phosphonate, sulfonic acid gives sulfonate.
onato
Modification to the name of an 'onate' anion when acting as a ligand: e.g.
phosphonato, sulfonato.
onic
Ending for the name of acids of the types RSO2OH or RPO(OH)2: e.g.
sulfonic acid, phosphonic acid (R = H, alkyl or aryl).
onite
Ending to designate salts and esters derived from 'onous' oxoacids.
onium
Ending for the name of a cation formed by hydron addition to a mononuclear
parent hydride: e.g. phosphonium.
ono
Termination for prefixes describing an oxoacid of connectivity 4 with one H
or alkyl group connected to the central atom: e.g. phosphono.
onous
Termination for the name of acids of the type PR(OH)2: e.g. phophonous
acid (R = H).
onoyl
Termination for prefixes indicating a substituent group of the type HX(O)<
(X = P or As): e.g. phosphonyl.
orane
Termination indicating a substituted derivative of the type XH5: e.g.
dichlorotriphenylphosphorane (X = P).
orous
Ending of a name of a molecular oxoacid of a central element in an
oxidation state lower than the highest, e.g. phosphorous, sulfurous.
oryl
Termination for prefix indicating a group of the type X(O): e.g. phosphoryl
(X = P).
triyl
Suffix composed of the suffix 'yl' and the multiplying prefix 'tri' indicating
the loss of three hydrogen atoms from the same or different atoms leaving a
residue which forms only three single bonds: e.g. boranetriyl, -B<; trisilane1,2,3-triyl, -SiH2SiHSiH2-; λ5-phosphanetriyl, -H2P<. (See also 'ylidyne'
and 'ylylidene').
m
m
co
Re
al
ion
vis
Termination for names of some substituent groups: e.g. hydroxy, carboxy.
Suffix denoting a substituent group: e.g. methyl, phosphanyl.
PA
C
yl
Pr
o
y
en
da
tio
ns
ocene
Suffix denoting a radical: e.g. methyl for CH3•.
Termination for non-systematic names of oxygenated cations: e.g. uranyl.
IU
ylene
Page 3 of 4
Obsolete suffix; replaced by 'diyl' (with the exception of methylene for
-CH2-, phenylene for -C6H4-).
DRAFT 2 April 2004
4
Suffix for name of a substituent group, formed by the loss of two hydrogen
atoms from the same atom of a parent chain or ring, forming a partner in a
double bond, i.e. =R not -R-.
ylidyne
Suffix for name of a substituent group formed by the loss of three hydrogen
atoms from the same atom, forming a partner in a triple bond. (See also
'ylylidene' and 'triyl').
ylylidene
Suffix for name of a substituent group formed by the loss of three hydrogen
atoms from the same or different atoms, forming a single bond and a double
bond. (See also 'ylidyne' and 'triyl').
yne
Ending indicating the presence of a triple bond between two atoms.
en
da
tio
ns
ylidene
m
a In the nomenclature of organic compounds, two terms designate the element of name
IU
PA
C
Pr
o
vis
ion
al
Re
co
m
formation at the end of a name, suffix and ending. Endings are: ane, ene and yne, etc.;
suffixes correspond to classes, e.g. carboxylic acids, amides, nitriles, etc.
DRAFT 2 April 2004
Page 4 of 4
m
m
co
henicosa
docosa
tricosa
triaconta
hentriaconta
pentatriaconta
tetraconta
octatetraconta
pentaconta
dopentaconta
hexaconta
heptaconta
octaconta
nonaconta
hecta
dicta
pentacta
kilia
dilia
Re
21
22
23
30
31
35
40
48
50
52
60
70
80
90
100
200
500
1000
2000
al
mono
di (bis) a
tri (tris)
tetra (tetrakis)
penta (pentakis)
hexa (hexakis)
hepta (heptakis)
octa (octakis)
nona (nonakis)
deca (decakis)
undeca
dodeca
trideca
tetradeca
pentadeca
hexadeca
heptadeca
octadeca
nonadeca
icosa
ion
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
en
da
tio
ns
Table IV Numerical prefixes
IU
PA
C
Pr
o
vis
a In the case of chelating ligands, the term 'bidentate' rather than 'didentate' is recommended.
Page 1 of 1
DRAFT 2 April 2004
Table V Geometrical and structural prefixes
s
These affixes are italicized and separated from the rest of the name by hyphens.
eight atoms bound into a regular antiprism
arachno
a boron structure intermediate between nido and hypho in degree of
openness
asym
asymmetrical
catena
a chain structure; often used to designate linear polymeric substances
cis
two groups occupying adjacent positions in a coordination sphere
closo
a cage or closed structure, especially a boron skeleton that is a
polyhedron having all faces triangular
cyclo
a ring structure. (Here, cyclo is used as a modifier indicating structure
and hence is italicized. In organic nomenclature, ‘cyclo’ is considered to
be part of the parent name since it changes the molecular formula. It is
therefore not italicized).
om
me
n
da
tio
n
antiprismo
ec
dodecahedro eight atoms bound into a dodecahedron with triangular faces
specifies the bonding of contiguous atoms of a ligand to a central atom
fac
three groups occupying the corners of the same face of an octahedron
hexahedro
eight atoms bound into a hexahedron (e.g. cube)
hexaprismo
twelve atoms bound into a hexagonal prism
hypho
an open structure, especially a boron skeleton, more closed than a klado
structure but more open than an arachno structure
icosahedro
twelve atoms bound into a triangular icosahedron
κ (kappa)
specifies the donor atoms in a ligand
klado
a very open polyboron structure
λ (lambda)
signifies, with its superscript, the bonding number, i.e. the sum of the
number of skeletal bonds and the number of hydrogen atoms associated
with an atom in a parent compound
Pr
ov
isi
o
na
lR
η (eta)
meridional; three groups occupying vertices of an octahedron so that
one is cis to the other two which are themselves mutually trans
µ (mu)
signifies that a group so designated bridges two or more coordination
centres
nido
a nest-like structure, especially a boron skeleton that is almost closed
IU
PA
C
mer
Page 1 of 2
DRAFT 2 April 2004
six atoms bound into an octahedron
pentaprismo
ten atoms bound into a pentagonal prism
quadro
four atoms bound into a quadrangle (e.g. square)
sym
symmetrical
tetrahedro
four atoms bound into an tetrahedron
trans
two groups occupying positions in a coordination sphere directly
opposite each other, i.e. in the polar positions of a sphere
triangulo
three atoms bound into a triangle
triprismo
six atoms bound into a triangular prism
IU
PA
C
Pr
ov
isi
o
na
lR
ec
om
me
n
da
tio
n
s
octahedro
DRAFT 2 April 2004
Page 2 of 2
Table VI
s
n
Element priority sequence
o
i
at
H
d
n
e
m
m
o
c
He
Li
Be
Ne
Na
Mg
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Xe
Cs
Ba
La
Lu
Hf
Ta
W
Re
Os
Ir
Pt
Rn
Fr
Ra
Ac
Lr
Rf
Db
Sg
Bh
Hs
Mt
l
a
B
C
N
O
F
Al
Si
P
S
Cl
Zn
Ga
Ge
As
Se
Br
Ag
Cd
In
Sn
Sb
Te
I
Au
Hg
Tl
Pb
Bi
Po
At
e
R
Ds
n
io
This element priority sequence is based on electronegativity considerations. The element columns (Groups 1 to 18 of the Periodic
Table) are connected by arrows leading from the less metallic elements to the more metallic elements. Of any two elements, that
reached later on following the arrow has the higher priority.
s
i
v
I
Page 1 of 1
C
A
P
U
o
r
P
DRAFT 2 April 2004
Table VII
Ligand abbreviations
s
n
Guidelines for the construction and use of ligand abbreviations are given in Section IR-4.4.4 and their use in the formulae of coordination complexes is described
in Section IR-9.2.3.4. Abbreviations are listed in alphabetical order but those beginning with a numeral are listed by the first letter of the abbreviation (e.g. 2,3,2-tet
appears under the letter 't').
o
i
at
d
n
e
m
m
o
c
Structural formulae of selected ligands are shown in Table VIII (numbered according to the present Table).
Number and
Systematic name
Other name (from which abbreviation derived)
abbreviation
1. 4-abu
4-aminobutanoato
2. Ac
acetyl
3. acac
2,4-dioxopentan-3-ido
4. acacen
2,2'-[ethane-1,2-diylbis(azanylylidene)]bis(4-oxopentan-3-ido)
5. ade
1H-purin-6-amine
6. ado
9-β-D-ribofuranosyl-9H-purin-6-amine
7. adp
adenosine 5'-diphosphato(3–)
8. aet
11. amp
Page 1 of 12
n
io
s
i
v
o
r
P
acetylacetonato
bis(acetylacetonato)ethylenediamine
adenine
adenosine
2-aminoethanethiolato
9. ala
10. ama
C
A
P
U
l
a
e
R
I
2-aminopropanoato
alaninato
2-aminopropanedioato
aminomalonato
adenosine 5'-phosphato(2–)
adenosine monophosphato
DRAFT 2 April 2004
12. [9]aneN3 (also tacn)
1,4,7-triazonane
13. [12]aneN4 (also cyclen)
1,4,7,10-tetraazacyclododecane
14. [l4]aneN4 (also cyclam)
1,4,8,11-tetraazacyclotetradecane
15. [18]aneP4O2
1,10-dioxa-4,7,13,16-tetraphosphacyclooctadecane
16. [9]aneS3
1,4,7-trithionane
17. [12]aneS4
l,4,7,10-tetrathiacyclododecane
18. arg
2-amino-5-carbamimidamidopentanoato
19. asn
2,4-diamino-4-oxobutanoato
20. asp
2-aminobutanedioato
21. atmp
[nitrilotris(methylene)]tris(phosphonato)(6–)
22. atp
adenosine 5'-triphosphato(4–)
23. 2,3-bdta
2,2',2",2'"-(butane-2,3-diyldinitrilo)tetraacetato
24. benzo-15-crown-5
2,3,5,6,8,9,11,12-octahydro-1,4,7,10,13-benzopentaoxacyclo-pentadecine
25. big
bis(carbamimidoyl)azanido
26. biim
2,2'-bi(1H-imidazole)-1,1'-diido
27. binap
o
i
at
d
n
e
m
m
o
c
1,4,7-trithiacyclononane
argininato
l
a
e
R
asparaginato
aspartato
aminotris(methylenephosphonato)
n
io
s
i
v
o
r
P
biguanid-3-ido
2,2'-biimidazolato
1,1'-binaphthalene-2,2'-diylbis(diphenylphosphane)
28. bn
29. bpy
C
A
P
U
s
n
1,4,7-triazacyclononane
butane-2,3-diamine
I
2,2'-bipyridine
DRAFT 2 April 2004
Page 2 of 12
s
n
30. 4,4'-bpy
4,4'-bipyridine
31. Bu
butyl
32. bzac
1,3-dioxo-1-phenylbutan-2-ido
33. bzim
benzimidazol-2-ido
34. Bz
benzyl
35. bztz
1,3-benzothiazole
36. cat
benzene-1,2-diolato
37. cbdca
cyclobutane-l,l-dicarboxylato
38. cdta
2,2',2",2'"-(cyclohexane-1,2-diyldinitrilo)tetraacetato
39. C5H 4Me
methylcyclopentadienyl
40. chxn (also, dach)
cyclohexane-1,2-diamine
41. cit
2-hydroxypropane-l,2,3-tricarboxylato
42. C5Me5 1
pentamethylcyclopentadienyl
43. cod
cycloocta-1,5-diene
44. cot
cycloocta-1,3,5,7-tetraene
45. Cp
Page 3 of 12
d
n
e
m
m
o
c
catecholato
l
a
e
R
n
io
s
i
v
o
r
P
cyclopentadienyl
46. cptn
47. 18-crown-6
C
A
P
U
cyclopentane-1,2-diamine
I
o
i
at
benzoylacetonato
1,4,7,10,13,16-hexaoxacyclooctadecane
DRAFT 2 April 2004
citrato
48. crypt-211
4,7,13,18-tetraoxa-1,10-diazabicyclo[8.5.5]icosane
cryptand 211
49. crypt-222
4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane
cryptand 222
50. Cy
cyclohexyl
cyclen (see [12]aneN4, No. 13)
2-amino-3-sulfanylpropanoato
52. cyt
4-aminopyrimidin-2(1H)-one
53. dabco
1,4-diazabicyclo[2.2.2]octane
cysteinato
cytosine
dach (see chxn, No. 40)
54. dbm
1,3-dioxo-1,3-diphenylpropan-2-ido
55. dea
2,2'-azanediylbis(ethanolato)
56. depe
ethane-1,2-diylbis(diethylphosphane)
57. diars
benzene-1,2-diylbis(dimethylarsane)
58. dien
N-(2-aminoethyl)ethane-1,2-diamine
59. [14]1,3-dieneN4
1,4,8,11-tetraazacyclotetradeca-1,3-diene
n
io
C
A
P
U
o
r
P
dibenzoylmethanato
diethanolaminato
1,2-bis(diethylphosphino)ethane
diethylenetriamine
[2,2-dimethyl-1,3-dioxolane-4,5-diylbis(methylene)]bis(diphenylphosphane)
61. diox
62. dipamp
l
a
e
R
s
i
v
60. diop
o
i
at
d
n
e
m
m
o
c
cyclam (see [14]aneN4, No. 14)
51. cys
s
n
1,4-dioxane
I
ethane-1,2-diylbis[(2-methoxyphenyl)phenylphosphane]
DRAFT 2 April 2004
'dimer of phenylanisylmethylphosphine'
Page 4 of 12
N,N-dimethylacetamide
64. dme
1,2-dimethoxyethane
65. dmf
N,N-dimethylformamide
66. dmg
butane-2,3-diylidenebis(azanolate)
67. dmpe
ethane-1,2-diylbis(dimethylphosphane)
68. dmpm
methylenebis(dimethylphosphane)
69. dmso
(methanesulfinyl)methane
70. dpm
2,2,6,6-tetramethyl-3,5-dioxoheptan-4-ido
71. dppe
ethane-1,2-diylbis(diphenylphosphane)
72. dppf
1,1'-bis(diphenylphosphanyl)ferrocene
73. dppm
methylenebis(diphenylphosphane)
74. dppp
propane-1,3-diylbis(diphenylphosphane)
75. dtmpa
[(phosphonatomethyl)azanediylbis(ethane-2,1diyl)dinitrilobis(methylene)]tetrakis(phosphonato)(10–)
diethylenetriaminepentakis(methylenephosphonato)
76. dtpa
2,2',2",2'"-[(carboxylatomethyl)azanediylbis(ethane-2,1-diyl)dinitrilo]tetraacetato
diethylenetriaminepentaacetato
78. edda
80. edtmpa
Page 5 of 12
I
C
A
P
U
o
i
at
d
n
e
m
m
o
c
dimethylglyoximato
1,2-bis(dimethylphosphino)ethane
bis(dimethylphosphino)methane
dimethyl sulfoxide
l
a
e
R
n
io
s
i
v
77. ea
79. edta
dimethylacetamide
s
n
63. dma
o
r
P
dipivaloylmethanato
1,2-bis(diphenylphosphino)ethane
bis(diphenylphosphino)methane
1,3-bis(diphenylphosphino)propane
2-aminoethanolato
ethanolaminato
2,2'-[ethane-1,2-diylbis(azanediyl)]diacetato
ethylenediaminediacetato
2,2',2",2'"-(ethane-1,2-diyldinitrilo)tetraacetato
ethylenediaminetetraacetato
[ethane-1,2-diyldinitrilotetrakis(methylene)]tetrakis(phosphonato)(8–)
ethylenediaminetetrakis(methylenephosphonato)
DRAFT 2 April 2004
s
n
81. egta
2,2',2",2'"-[ethane-1,2-diylbis(oxyethane-2,1-diylnitrilo)]tetraacetato
82. en
ethane-1,2-diamine
83. Et
ethyl
84. Et2dtc
N,N-diethylcarbamodithioato
85. fod
6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-dioxooctan-4-ido
86. fta
l,1,l-trifluoro-2,4-dioxopentan-3-ido
87. gln
2,5-diamino-5-oxopentanoato
88. glu
2-aminopentanedioato
89. gly
aminoethanoato, or 2-aminoacetato
90. gua
2-amino-9H-purin-6(1H)-one
91. guo
2-amino-9-β-D-ribofuranosyl-9H-purin-6(1H)-one
92. hdtmpa
[(hexane-1,6-diyldinitrilo)tetrakis(methylene)]tetrakis(phosphonato)(8–)
hexamethylenediaminetetrakis(methylenephosphonato)
93. hedp
1-hydroxyethane-1,1-bis(phosphonato)(4–)
1-hydroxyethane-1,1-diphosphonato
94. hfa
1,1,1,5,5,5-hexafluoropentane-2,4-dioxopentan-3-ido
hexafluoroacetylacetonato
95. his
2-amino-3-(imidazol-4-yl)propanoato
histidinato
C
A
P
U
d
n
e
m
m
o
c
N,N-diethyldithiocarbamato
trifluoroacetylacetonato
glutaminato
l
a
e
R
s
i
v
o
r
P
glutamato
glycinato
guanine
guanosine
N,N,N',N',N",N"-hexamethylphosphoric triamide
97. hmta
98. ida
o
i
at
n
io
96. hmpa
ethylene glycol-bis(2-aminoethyl)-N,N,N',N'-tetraacetic acid
I
1,3,5,7-tetraazatricyclo[3.3.1.13,7]decane
hexamethylenetetraamine
2,2'-azanediyldiacetato
iminodiacetato
DRAFT 2 April 2004
Page 6 of 12
99. ile
2-amino-3-methylpentanoato
100. im
imidazol-1-ido
101. isn
pyridine-4-carboxamide
102. leu
2-amino-4-methylpentanoato
103. lut
2,6-dimethylpyridine
104. lys
2,6-diaminohexanoato
105. mal
2-hydroxybutanedioato
106. male
(Z)-butenedioato
107. malo
propanedioato
108. Me
methyl
109. 2-Mepy
2-methylpyridine
110. met
2-amino-4-(methylsulfanyl)butanoato
111. mnt
1,2-dicyanoethene-1,2-dithiolato
112. napy
1,8-naphthyridine
113. nbd
bicyclo[2.2.1]hepta-2,5-diene
norbornadiene
pyridine-3-carboxamide
nicotinamide
114. nia
115. nmp
116. nta
Page 7 of 12
C
A
P
U
o
i
at
isonicotinamide
d
n
e
m
m
o
c
leucinato
lutidine
lysinato
malato
l
a
e
R
n
io
s
i
v
maleato
malonato
methioninato
maleonitriledithiolato
o
r
P
N-methylpyrrolidine
I
s
n
isoleucinato
2,2',2"-nitrilotriacetato
DRAFT 2 April 2004
117. oep
2,3,7,8,12,13,17,18-octaethylporphyrin-21,23-ido
118. ox
ethanedioato
119. pc
phthalocyanine-29,31-diido
120. 1,2-pdta
2,2',2",2'"-(propane-1,2-diyldinitrilo)tetraacetato
121. 1,3-pdta
2,2',2",2'"-(propane-1,3-diyldinitrilo)tetraacetato
122. Ph
phenyl
123. phe
2-amino-3-phenylpropanoato
124. phen
1,10-phenanthroline
125. pip
piperidine
126. pn
propane-1,2-diamine
127. pmdien
2,2'-(methylazanediyl)bis(N,N-dimethylethanamine)
128. ppIX
2,18-bis(carboxyethyl)-3,7,12,17-tetramethyl-8,13-divinylporphyrin-21,23-diido
protoporphyrinato IX
129. pro
pyrrolidine-2-carboxylato
prolinato
130. ptn
pentane-2,4-diamine
131. py
pyridine
1,3-propylenediaminetetraacetato
phenylalaninato
l
a
e
R
C
A
P
U
s
i
v
o
r
P
N,N,N',N",N"-pentamethyldiethylenetriamine
pyrazine
133. pz
134. qdt
d
n
e
m
m
o
c
1,2-propylenediaminetetraacetato
n
io
132. pyz
s
n
o
i
at
oxalato
1H-pyrazol-1-ido
I
quinoxaline-2,3-dithiolato
DRAFT 2 April 2004
Page 8 of 12
135. quin
quinolin-8-olato
136. sal
2-hydroxybenzoato
salicylato
137. salan
2-[(phenylimino)methyl]phenolato
salicylideneanilinato
138. saldien
2,2'-[azanediylbis(ethane-2,1-diylazanylylidenemethanylylidene)]diphenolato
bis(salicylidene)diethylenetriaminato
139. salen
2,2'-[ethane-1,2-diylbis(azanylylidenemethanylylidene)]diphenolato
bis(salicylidene)ethylenediaminato
140. salgly
N-(2-oxidobenzylidene)glycinato
141. salpn
2,2'-[propane-1,2-diylbis(azanylylidenemethanylylidene)]diphenolato
142. saltn
2,2'-[propane-1,3-diylbis(azanylylidenemethanylylidene)]diphenolato
143. sdta
2,2',2",2'"-(1,2-diphenylethane-1,2-diyldinitrilo)tetraacetato
144. sep 2
1,3,6,8,10,13,16,19-octaazabicyclo[6.6.6]icosane
145. ser
2-amino-3-hydroxypropanoato
146. stien 3
1,2-diphenylethane-1,2-diamine
tacn (see [9]aneN3, No. 12)
o
i
at
d
n
e
m
m
o
c
salicylideneglycinato
l
a
e
R
n
io
s
i
v
o
r
P
bis(salicylidene)propylenediaminato
bis(salicylidene)trimethylenediaminato
stilbenediaminetetraacetato
serinato
147. tap
propane-1,2,3-triamine
148. tart
2,3-dihydroxybutanedioato
tartrato
ethenetetracarbonitrile
tetracyanoethylene
2,2'-(cyclohexa-2,5-diene-1,4-diylidene)bis(propane-1,3-dinitrile)
tetracyanoquinodimethane
149. tcne
150. tcnq
151. tdt
Page 9 of 12
I
C
A
P
U
s
n
1,2,3-triaminopropane
4-methylbenzene-1,2-dithiolato
DRAFT 2 April 2004
1
s
n
152. tea
2,2',2"-nitrilotris(ethanolato)
triethanolaminato
153. terpy
2,2':6',2"-terpyridine
terpyridine
154. 2,3,2-tet
N,N'-bis(2-aminoethyl)propane-1,3-diamine
1,4,8,11-tetraazaundecane
155. 3,3,3-tet
N,N' -bis(3-aminopropyl)propane-1,3-diamine
156. tetren
N,N' -(azanediyldiethane-2,1-diyl)bis(ethane-1,2-diamine)
157. tfa
trifluoroacetato
158. thf
oxolane
159. thiox
1,4-oxathiane
160. thr
2-amino-3-hydroxybutanoato
161. tht
thiolane
162. thy
5-methylpyrimidine-2,4(1H,3H)-dione
163. tmen
N,N,N',N' -tetramethylethane-1,2-diamine
164. tmp
5,10,15,20-tetrakis(2,4,6-trimethylphenyl)porphyrin-21,23-diido
5,10,15,20-tetramesitylporphyrin-21,23-diido
165. tn
propane-1,3-diamine
trimethylenediamine
166. Tol (o-, m- or p-)
2-, 3- or 4-methylphenyl
tolyl (o-, m- or p-)
hydridotris(pyrazolido-N)borato(1–), or tris(1H-pyrazol-1-yl)boranuido
hydrotris(pyrazolyl)borato
hydridotris(3,5-dimethylpyrazolido-N)borato(1–)
hydrotris(3,5-dimethylpyrazolyl)borato
167. Tp
168. Tp' 4
169. tpp
I
C
A
P
U
o
i
at
d
n
e
m
m
o
c
1,5,9,13-tetraazatridecane
tetraethylenepentamine
tetrahydrofuran
l
a
e
R
n
io
s
i
v
o
r
P
thioxane
threoninato
tetrahydrothiophene
thymine
5,10,15,20-tetraphenylporphyrin-21,23-diido
DRAFT 2 April 2004
Page 10 of 12
1
170. tren
N,N-bis(2-aminoethyl)ethane-1,2-diamine
tris(2-aminoethyl)amine
171. trien
N,N'-bis(2-aminoethyl)ethane-l,2-diamine
triethylenetetramine
172. triphos 5
[(phenylphosphanediyl)bis(ethane-2,1-diyl)]bis(diphenylphosphane)
173. tris
2-amino-2-(hydroxymethyl)propane-1,3-diol
174. trp
2-amino-3-(1H-indol-3-yl)propanoato
175. tsalen
2,2'-[ethane-1,2-diylbis(azanylylidenemethanylylidene)]dibenzenethiolato
176. ttfa
4,4,4-trifluoro-1,3-dioxo-1-(2-thienyl)butan-2-ido
177. ttha
[2,2',2'',2'''-(ethane-1,2-diylbis{[(carboxylatomethyl)azanediyl]ethane-2,1diyl}nitrilo)]tetraacetato
178. ttp
5,10,15,20-tetrakis(4-methylphenyl)porphyrin-21,23-diido
179. tu
thiourea
180. tyr
2-amino-3-(4-hydroxyphenyl)propanoato
181. tz
1,3-thiazole
182. ura
pyrimidine-2,4(1H,3H)-dione
183. val
2-amino-3-methylbutanoato
Footnotes
1.
C
A
P
U
s
n
o
i
at
d
n
e
m
m
o
c
aminotris(hydroxymethyl)methane
tryptophanato
bis(thiosalicylidene)ethylenediaminato
thenoyltrifluoroacetonato
l
a
e
R
n
io
s
i
v
o
r
P
triethylenetetraminehexaacetato
5,10,15,20-tetra-p-tolylporphyrin-21,23-diido
tyrosinato
thiazole
uracil
valinato
The use of the abbreviation Cp* for pentamethylcyclopentadienyl is discouraged. It can lead to confusion because the asterisk, *, is also used to represent
an excited state, an optically active substance, a radioactive substance, etc.
I
Page 11 of 12
DRAFT 2 April 2004
1
2.
The abbreviation derives from the non-systematic name sepulchrate which incorrectly implies that the ligand is anionic.
3.
The abbreviation derives from the non-systematic name stilbenediamine which incorrectly implies the presence of a C=C double bond in the ligand.
4.
The use of Tp' is preferred to Tp* for the reasons given in Note 1. A general procedure for abbreviating substituted hydridotris(pyrazolido-N)borate ligands
has been proposed {see S. Trofimenko, Chem. Rev., 93, 943 (1993)}. For example, Tp' becomes TpMe2, the superscript denoting the methyl groups at the
3- and 5-positions of the pyrazolyl rings.
5.
The abbreviation triphos should not be used for the ligand PhP(CH2PPh2)3.
s
n
o
i
at
l
a
d
n
e
m
m
o
c
e
R
n
io
s
i
v
I
C
A
P
U
o
r
P
DRAFT 2 April 2004
Page 12 of 12
1
Table VIII
Structural formulae of selected ligands (numbered according to Table VII)
O
H 2N
CO2
O
O
Me
Me
Me
-
Me
-
3
OH
6
5
H
H
-
NH2
CO2
-
-
9
O 2C
O
- O
P
O
NH HN
HN
CO2
tio
n
H
7
NH2
-
N
N
O
NH HN
P
O
H
- O
14
ov
13
O
HP
PH
HP
S
IU
PA
C
Pr
PH
S
H 2N
S
S
S
O
15
16
17
NH2
O
H
N
CO2
NH
Page 1 of 10
S
S
NH2
-
18
CO2
H 2N
19
DRAFT 2 April 2004
-
OH
OH
O
-O
isi
o
N H H
N
NH HN
NH HN
12
H
- O
10
na
N
H
NH
P
O
lR
8
O
ec
Me
OH
NH2
NH2
S
OH
O
O
HO
N H H
N
OH
O
da
N
H
N
N H H
N
N
N
om
me
n
N
NH2
N
N
N
O
4
NH2
NH2
Me
s
Me
1
-
-
N
N
H
11
2
O
-
O-
O
P
P
-
O 2C
CO2
O
-
O
N
-
O
P
20
21
da
-O
N HH
O
O
O
O
- P O P- O P- O
O O
O
O
om
me
n
N
N
H
OH
OH
H
-O
2CCH2
2CCH2
N
ec
lR
O
O
O
N
N
N
ov
NH
NH
N
NH2
-
Me
PPh2
PPh2
N
NH2
H 2N
-
Pr
Me
26
PA
C
CH2CO2
25
isi
o
24
H 2N
na
O
N
N
CH2CO2
23
O
N
Me
Me
22
-
O
O-
NH2
N
-
tio
n
NH2
s
-O
28
27
O
Ph
O
-
Me
N
N
S
-
29
33
35
IU
32
N
DRAFT 2 April 2004
Page 2 of 10
3
CO2
CO2
-
-
-
37
36
N
O2CCH2
O 2C
-
N
CH2CO2
41
O
O
O
O
46
N
NH2
NH2
O
O
N
HS
CO2
O
51
N
-
-
O
Ph
ov
H
N
NH2
53
52
N
H
PEt2
AsMe2
55
56
O
-
57
H
N
O
N
NH
Pr
H 2N
N
H
AsMe2
isi
o
54
PA
C
IU
Page 3 of 10
PPh2
PPh2
O
HN
H
58
60
59
MeO
Ph
NO
O
Me
Me
P
P
Me
N
62
Me
Me
Ph
OMe
N
N
PEt2
na
Ph
O
O
lR
49
-
ec
O
O
O
48
47
O
O
N
O
O
N
om
me
n
O
da
O
H 2N
-
O 2C OH
CH2CO2
O2CCH2
O
-
-
38
H 2N
CO2
s
O
-
tio
n
O
63
DRAFT 2 April 2004
NO
66
-
4
O
PMe2
O
PMe2
Me
Me
-
PMe2
70
68
67
Me
Me
s
Me Me
PPh2
PPh2
tio
n
PMe2
PPh2
PPh2
Fe
PPh2
PPh2
PPh2
74
73
72
om
me
n
71
da
PPh2
O
-
O
O
O-
O
-
P
P
N
O-
O
N
N
-O
P
-O
O
O-
O
P
ec
P
O
-
O
-
O
-
O2CCH2
CH2CO2
N
N
N
na
-
CH2CO2
-
CH2CO2
O
NH2
O2CCH2
N
Pr
N
IU
PA
C
79
O2CCH2
H
N
N
H
-
O
-
P
-O
N
CH2CO2
-O
O
O-
O
P
CH2CO2
-
CH2CO2
78
O
CH2CO2
-
-
77
ov
-
-
isi
o
CH2CO276
lR
75
P
O
N
O-
-
P
O
80
O
-
O
CH2CO2
-
O2CCH2
O
O
N
CH2CO2-
N
CH2CO2
81
DRAFT 2 April 2004
Page 4 of 10
5
Me
Et
N
S
O
-
-
Me
H 2N
Me
Et
O
84
NH2
O
C 3F 7
-
O
CO2
CF3
O
86
85
87
s
Me
tio
n
S
O
CO2
89
P
-O
N
-
O
P
O
O
O
P
-
-
92
P
P
CF3
-
na
N
HN
94
-
N
H
CO2
ov
O 2C
isi
o
CF3
O-
O
O
- O
O
N
P
-
Me2N
NH2
NMe 2
NMe 2
N
96
95
NH2
-
CO2
N
-
N
99
PA
C
IU
Page 5 of 10
O
100
NH2
NH2
CO2
N
102
101
DRAFT 2 April 2004
-
Me
N
103
N
N
97
-
Pr
98
H
HO
93
O
O
O
91
OH
OH
H
P
Me
O
lR
-
N HH
O-
O
O
OO
O
N
OH
N
O-
H 2N
N
H
90
-
-O
N
ec
O
H 2N
O
N
HN
N
HN
H 2N
-
88
O
-
om
me
n
HO 2C
O
da
NH2
O
Me
-
NH2
CO2
-
O 2C
104
105
MeS
CO2
CN
-
S
110
-
CO2
106
S
NH2
CO2
OH
-
-
-
N
CN
-
s
-
6
107
N
tio
n
CO2
CO2
-
da
H 2N
CO2
-
112
om
me
n
111
O
NH2
N
N
Me
114
113
lR
Et
ec
115
Et
-
O2CCH2
-
N
N
CH2CO2
na
CH2CO2
Et
-
N
-
N
Et
N
isi
o
-
O
O
-
118
Et
117
Pr
ov
Et
O
Et
Et
116
O
N
IU
PA
C
N
N
-
N
-
N
N
-
N
-
O2CCH2
N
O2CCH2
N
Me
N
CH2CO2
-
CH2CO2
120
119
DRAFT 2 April 2004
Page 6 of 10
7
N
N
CO2
Ph
NH2
CH2CO2
N
121
124
NH2
Me
N
Me
NH2
127
Me
Me
H
N
N
om
me
n
126
125
NMe 2
da
Me2N
N
H
N-
N
123
s
O2CCH2
CH2CO2
tio
n
-
CH2CO2
-
O
H 2N
NH2
O-
-N
129
Me
Me
CH2CH2CO2H
lR
HO2CCH2CH2
128
na
N
isi
o
-
133
N
PA
C
Page 7 of 10
-
-
136
H
N
N
O
Ph
-
-
137
N
N
-
O
138
O
N
-
139
IU
O
S
-
-
135
N
N
OH
Pr
O
S
134
CO2
ov
132
N
N
N
N
130
ec
N
DRAFT 2 April 2004
O
8
N
N
-
O
O
N
-
-
O
s
O 2C
141
O
N
-
-
da
N
tio
n
140
O
-
O2CCH2
CH2CO2
N
N
Ph
Ph
-
om
me
n
142
N
CH2CO2
O2CCH2
143
ec
lR
HO
CO2
-
isi
o
CO2
-
O 2C
PA
C
ov
S
Me
HN
NH2
Ph
146
NC
CN
NC
CN
-
150
148
O
-
-
N
S
-
-
O
N
O
-
152
N
N
153
IU
151
H
N
N
OH
Pr
147
HN
NH2
OH
NH2
NH
Ph
na
145
NH2
HN
144
NH2
NH2
NH
DRAFT 2 April 2004
Page 8 of 10
9
H 2N
H
N
H
N
H 2N
NH2
155
H
N
H 2N
H
N
N
H
NH2
O
NH2
O
CH3
S
OH
159
161
160
NH
om
me
n
CO2
da
156
S
NH2
s
154
H
N
tio
n
H
N
N
H
Me
Me
Me
N
Me
163
-
N
-
N
N
Me
N
Me
167
Me
ov
N
-
N
PA
C
Pr
BH–
N
Me
N
-
N
169
IU
Page 9 of 10
N
N
isi
o
Me
164
N
N
Me
na
Me
Me
ec
Me
lR
Me
DRAFT 2 April 2004
N
O
162
Me
N
Me
10
Ph
NH2
NH2
N
H
NH2
P
Ph
171
Ph
P
Ph
tio
n
170
Ph
s
H 2N
N
H 2N
P
H
N
172
OH
HN
NH2
da
H 2N
OH
CO2
om
me
n
OH
174
173
O
N
N
S
175
-
CH2CO2
CH2CO2
N
N
N
O2CCH2
ec
176
-
N
CH2CO2
CH2CO2
na
CH2CO2
isi
o
177
N
H 2N
N
Me
-
N
N
178
NH2
179
Me
Pr
PA
C
-
Me
ov
S
Me
lR
-
CF3
-
-
-
O
S
-
S
-
O
HO
CO2
-
S
181
NH
CO2
N
H
182
-
O
183
IU
180
NH2
N
NH2
DRAFT 2 April 2004
Page 10 of 10