* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Phosphodiesterase 5 inhibitors: are they cardioprotective?
Neuropharmacology wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Psychopharmacology wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
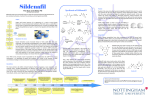
Review Cardiovascular Research (2009) 83, 204–212 doi:10.1093/cvr/cvp170 Phosphodiesterase 5 inhibitors: are they cardioprotective? Thorsten Reffelmann1,2* and Robert A. Kloner2 1 Klinik und Poliklinik für Innere Medizin B, Universitätsklinikum der Ernst-Moritz-Arndt-Universität Greifswald, FriedrichLöffler-Str. 23 a, 17475 Greifswald, Germany; and 2The Heart Institute, Good Samaritan Hospital, Division of Cardiology, Keck School of Medicine, University of Southern California, 1225 Wilshire Boulevard, Los Angeles, CA 90017-2395, USA Received 7 April 2009; revised 20 May 2009; accepted 25 May 2009; online publish-ahead-of-print 27 May 2009 Time for primary review: 16 days KEYWORDS Phosphodiesterase 5; Sildenafil; Vardenafil; Tadalafil; Preconditioning A growing body of animal studies provides evidence for potential cardioprotective effects of inhibitors of the enzyme phosphodiesterase isoform 5. Infarct size reduction by administration of phosphodiesterase 5 inhibitors was described in various experimental models of ischaemia and reperfusion. Furthermore, potential beneficial effects were demonstrated in experimental models of congestive heart failure and left ventricular hypertrophy. Some of the observed effects resemble the basic mechanisms of ischaemic pre-conditioning, mimicking both acute and delayed effects. Other effects may be due to action on systemic and cardiac haemodynamics. Mechanisms and signalling pathways, characterized in some of the experimental models, appear to be complex: for instance, the rate of cyclic guanosine monophosphate (cGMP) synthesis and the functional compartmentalization of intracellular cGMP metabolism as well as interaction with ß-adrenergic and nitric oxide signalling may influence effects in different experimental settings. In this review, we discuss mechanisms, signalling pathways, and experimental limitations and touch on considerations for translation into potentially useful applications in the clinical arena. 1. Introduction To date, three pharmacological inhibitors of the enzyme phosphodiesterase (PDE) isoform 5 (PDE 5) have been approved for the treatment of erectile dysfunction: sildenafil, vardenafil, and tadalafil. While sildenafil was initially developed in an effort to find a novel pharmacological class with anti-anginal properties, the side effect of enhancing penile erections, as reported by a substantial number of volunteers in the first clinical investigations, soon became the new focus of research. Anti-anginal effects, however, turned out to be limited in these clinical studies.1 Shortly after the approval of sildenafil for the treatment of erectile dysfunction in 1998, potential cardiovascular effects of sildenafil again attracted increasing attention. Both the popular press and scientific journals published reports about adverse cardiovascular events, such as myocardial infarction, in temporal relation to sildenafil intake,2–4 which initiated a vigorous debate about the cardiovascular safety of sildenafil. Investigations of potential side effects of sildenafil2,5–9 were followed by systematic statistical analyses of cardiovascular events related to sildenafil, vardenafil, and tadalafil intake, in comparison to control populations. These studies did not support a cardiovascular risk specifically attributable to the * Corresponding author. Tel: þ49 3834 86 7574; fax: þ49 3834 86 6657. E-mail address: [email protected] pharmacological action of PDE 5 inhibitors.10–15 It became rather obvious that many of the patients suffering from erectile dysfunction are characterized by a high cardiovascular risk profile which may predispose them to an increased rate of adverse cardiovascular events.16–20 Following this extensive debate on potential cardiovascular side effects, research focus again shifted to the field of cardioprotection.21 A growing body of experimental animal studies provided evidence for infarct size reducing effects in myocardial ischaemia and reperfusion, mimicking in part mechanisms of ischaemic pre-conditioning. Furthermore, potential beneficial effects were also demonstrated in experimental models of congestive heart failure and left ventricular hypertrophy. The following review summarizes recent studies, predominantly from animal research, investigating a potential cardioprotective profile of PDE 5 inhibitors, discusses mechanisms, perspectives, and experimental limitations of these effects, and touches on considerations for translation into clinically meaningful indications. 2. The cyclic guanosine monophosphate – phosphodiesterase 5 pathway 2.1 Basic mechanisms and effects Phosphodiesterase is a family of enzymes catalyzing the breakdown of 30 ,50 -cyclic nucleotide monophosphates, Published on behalf of the European Society of Cardiology. All rights reserved. & The Author 2009. For permissions please email: [email protected]. Phosphodiesterase 5 inhibitors 205 Figure 1 Schematic illustrating regulation of cGMP-synthesis and breakdown, the action of phosphodiesterases, and potential external factors, such as heart failure, diabetes, atherosclerosis, or adrenergic drive, that could have significant influence on the complex interaction of various signal pathways. such as cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), which serve as important intracellular second messengers. Isoforms 5 and 6 are specific for the hydrolysis of cGMP, whereas PDE 3 and 4 catalyze the breakdown of cAMP specifically. Phosphodiesterase 1 and 2 hydrolyze both cAMP and cGMP. As a consequence, in tissue containing significant activity of PDE 5, inhibition of PDE 5 should result in slowed breakdown of the second messenger cGMP.1 Therefore, the rate of cGMP production will determine the final functional effects of PDE 5 inhibitors. Cyclic GMP is synthesized by guanylyl cyclases in response to endo- and exogenous stimuli: nitric oxide (NO), a product of the enzyme NO synthase, activates the soluble guanylyl cyclase. Natriuretic peptides activate the particulate guanylyl cyclase22 (Figure 1). cGMP activates a pattern of specific protein kinases, in particular protein kinase G, which serve as main effectors of cGMP, for example via reducing intracellular calcium.23 Interestingly, a functional compartmentalization of cGMP synthesized by the soluble and particulate guanylyl cyclase was suggested in cardiomyocytes with differential effects on ß-adrenergic modulation of cardiac contractility and differential control of cGMP-breakdown by PDE 5 inhibitors.24 These findings need further investigation as they might explain some of the unresolved issues in understanding the cardiovascular effects of PDE 5 inhibitors. 2.2 Tissue distribution of PDE 5: the basis for effects in vivo PDE 5 is found in high concentration in vascular smooth muscle cells of the corpora cavernosa of the penis, in smooth muscle cells of the peripheral arterial and venous vessels as well as coronary and pulmonary circulation, and in platelets.25 During sexual stimulation, NO is released from non-adrenergic, non-cholinergic nerve endings and endothelial cells in the corpora cavernosa. As a consequence, arterial smooth muscle cells in penile arteries relax due to increased cGMP-synthesis, which results in increased blood within the tunica albuginea, and hence a penile erection. This may be intensified and prolonged by PDE 5 inhibitors, slowing the breakdown of cGMP and enhancing smooth muscle relaxation and vasodilatation of penile blood vessels. Similarly, PDE 5 inhibitors may reduce vascular tone in the peripheral, pulmonary, or coronary circulation. In healthy volunteers, oral administration of sildenafil resulted in lowering of systolic and diastolic blood pressure by 7–10 mmHg in a dose-independent manner.26 Similar effects were described after vardenafil or tadalafil.11,27 Thus, under baseline conditions, vasodilatory effects of PDE 5 inhibitors are modest; however, when PDE 5 inhibitors and NO donors are administered simultaneously, which is absolutely contra-indicated, life-threatening hypotension may occur. Again, the degree of cGMP-production 206 determines functional effects of PDE 5 inhibitors. In the pulmonary circulation, vasodilatory effects were found to be beneficial in the treatment of primary arterial pulmonary hypertension, which finally led to the approval of oral sildenafil for this indication.28,29 Using aortic rings as an experimental model for the peripheral and—with limitations—also the coronary circulation, vasodilatory effects, accompanied by cGMP-accumulation, were demonstrated.25 According to measurements by Herrman et al.,7 using intracoronary Doppler-wire technique in patients during coronary angiography, coronary flow after sildenafil intake increased by 13% in stenosed coronary arteries as well as angiographically normal arteries. In most studies, PDE 5 inhibitors did not exacerbate myocardial ischaemia during exercise stress testing, but appeared to be neutral or slightly beneficial.30,31 Also, some anti-aggregatory effects on platelets, which also contain PDE 5, were described in these studies.31 2.3 PDE 5 activity in cardiomyocytes When discussing potential direct cardioprotective effects of PDE 5 inhibitors, a crucial issue is the question whether PDE 5 is expressed in ventricular cardiomyocytes. This issue has remained controversial. Initial investigations did not provide evidence for the presence of significant PDE 5 activity in ventricular cardiomyocytes. The cGMPhydrolyzing activity found in ventricular tissue appeared to be mainly due to other isoforms of PDE.25 In the study by Wallis et al.,25 no modulating effect of sildenafil on contractility of isolated trabeculae carneae was observed, which also argued against a functional role of PDE 5 in ventricular cardiomyocytes. However, in contrast to the investigations by Wallis et al., several publications suggested significant PDE 5 activity in canine, and murine ventricular cardiomyocytes32,33 and also expression of PDE 5 mRNA in human cardiomyocytes.34 In human atrial tissue, treatment with sildenafil resulted in increased levels of cGMP,35 which might also indicate significant activity of PDE 5 in atrial cardiomyocytes. It appears doubtful whether contamination with PDE 5 from vascular cells, as initially surmised to be the reason for the detection of PDE 5 activity in cardiac ventricular tissue preparations, is the sole explanation for the described discrepancies regarding the presence of PDE 5 in ventricular cardiomyocytes. An increasing number of studies reported functional effects of PDE 5 inhibition on cardiac contractility. In the study by Senzaki et al.,32 the presence of PDE 5 in canine cardiomyocytes was demonstrated by immunohistochemistry, and in parallel experiments, a modulatory effect of PDE 5 inhibition on ß-adrenergic modification of cardiac inotropy was observed. In murine hearts and isolated cardiomyocytes, contractility was reduced by sildenafil only when these hearts were stimulated with isoproterenol, but substantially less under baseline conditions.24,36 Interestingly, in mice with pharmacological inhibition of the enzyme NO synthase or transgenic mice lacking NO synthase 3, sildenafil did not exhibit any modulatory effects on ß-adrenergic modulation of cardiac contractility.36 In humans, contractility indices as assessed by echocardiography and blood pressure measurements during dobutamine infusion were significantly reduced after sildenafil intake, pointing to modulatory effects of PDE 5 inhibition also in the human heart.37 In investigations on functional T. Reffelmann and R.A. Kloner compartmentalization of cGMP derived from soluble and particulate guanylyl cyclase, Takimoto et al.24 found suppression of isoproterenol-stimulated increase in contractility by sildenafil which was prevented by pre-treatment with an inhibitor of protein kinase G, and also by inhibition of the NO synthase. However, atrial natriuretic peptide, an activator of the particulate guanylyl cyclase, did not alter isoproterenol-induced changes of contractility, and unlike sildenafil treatment it was accompanied by pronounced increase in myocardial cGMP. Other experimental models suggested that the ‘particulate cGMP-pool’ is under exclusive control of PDE 2 and, in contrast to the soluble cGMP-pool, not under control of PDE 5.38 In transgenic mice lacking NO synthase 3 activity or mice with chronic pharmacological inhibition of NO synthase, the typical co-localization of PDE 5A and z-band striations of the sarcomere, as visualized by immunostaining in normal wild-type mice, disappeared, and a diffuse PDE 5A staining without preference to the z-band was apparent.36 Similar translocation of PDE 5A activity away from z-bands during the development of heart failure in a canine tachycardia-induced heart failure model was observed by Senzaki et al.,32 which was accompanied by diminished modulatory effect of sildenafil on ß-adrenergic effects. These findings suggest a complex interaction of various pathways, including ß-adrenergic signalling, NO, and natriuretic peptide, and separate functional compartments of cGMP with differential functionality under regulation of distinct enzymatic patterns, which might in part explain some of the apparently contradictory results with respect to cardioprotective properties of PDE 5 inhibitors. 3. PDE 5 inhibitors in experimental myocardial ischaemia and reperfusion 3.1 PDE 5 inhibitors prior to ischaemia 3.1.1 Infarct size The landmark study, suggesting powerful infarct size reducing effects of sildenafil in an experimental model of coronary artery occlusion and reperfusion in rabbits, was published by Ockaili R et al.39 in 2002. In this study, 0.7 mg/kg sildenafil, administered as an intravenous bolus, 30 min prior to 30 min of coronary artery occlusion followed by 180 min of reperfusion, reduced infarct size by 68% (10.8 + 0.9% of the risk area vs. 33.8 + 1.7% in the control group). Administration of sildenafil 24 h prior to ischaemia resulted in 41% reduction of infarct size. 5Hydroxydecanoate, a blocker of the mitochondrial ATPsensitive potassium channel, abolished these infarct-size reducing effects. As opening of ATP-sensitive potassium channels is regarded as a key step in signalling of ischaemic pre-conditioning, sildenafil might be termed as a preconditioning mimetic agent. In this study, intravenous administration of sildenafil resulted in a substantial drop of arterial blood pressure prior to ischaemia (238.8% decline in mean arterial pressure within 2 min), which then returned to baseline within 5 min. During coronary occlusion, haemodynamics were not significantly different between groups in this experimental study. In 2001, Das S et al.40 had published a study, investigating the effect of sildenafil on infarct size in an isolated working-heart preparation in rats. In these investigations, Phosphodiesterase 5 inhibitors 0.05 mg/kg sildenafil resulted in significant infarct size reduction, but with higher doses of sildenafil substantial exacerbation of myocardial necrosis was observed over a very narrow dosing range (0.1–0.5 mg/kg sildenafil). Dosedependent effects of sildenafil on infarct size and ventricular function during reperfusion in working rat hearts were also described in a study by du Toit et al.41 In that study, higher doses of sildenafil were associated with decreased contractility during reperfusion. A similar experimental protocol, as used by Ockailli et al., was tested in another set of experiments from our group: 1.45 mg/kg sildenafil, administered intravenously over 5 min 30 min prior to 30 min of coronary artery occlusion in open chest rabbits, followed by 3 h of reperfusion did not result in significant infarct size reduction nor in exacerbation of myocardial necrosis.42 After a small blood pressure drop prior to ischaemia (217 to 219 mmHg for systolic and diastolic blood pressure, respectively), haemodynamics during ischaemia were not significantly different between groups in this study. Similarly, zones of no-reflow, regional myocardial blood flow in ischaemic and non-ischaemic tissue, and incident arrhythmias were not significantly different. The reasons for these discrepancies have not been well understood, as very parallel experimental protocols were used. Vardenafil was also tested in a similar open-chest rabbit model of regional myocardial ischaemia, followed by reperfusion: 0.014 mg/kg vardenafil, given 30 min prior to coronary artery occlusion resulted in 58% of infarct size reduction (14.3 + 2.2% of the risk area in comparison to 33.8 + 1.3% in control animals).43 This effect was blocked by an inhibitor of the mitochondrial ATP-sensitive potassium channel, but not by an inhibitor of the sarcolemmal ATP-sensitive potassium channel. Vardenafil administration caused a rapid decrease in blood pressure (215% in mean arterial blood pressure) within 5 min along with increasing heart rate. Over the next 10 min blood pressure increased and heart rate decreased, but did not return to baseline. Again, these experiments were interpreted as a pre-conditioningmimetic action of the PDE 5 inhibitor vardenafil, resulting in marked attenuation of myocardial necrosis.44 Tadalafil, a long-acting inhibitor of PDE 5, was tested in rats. Treatment with tadalafil (10 mg/kg by gastric gavage) 2 h prior to coronary artery occlusion for 30 min followed by 3 h of reperfusion significantly reduced infarct size (41.7 + 2.4 vs. 54.0 + 3.4% of the risk area).45 In these investigations, mean arterial blood pressure was significantly lower in tadalafil-treated animals in comparison with controls, which may provide an explanation for infarct size reduction as a consequence of reduced cardiac workload during ischaemia. No other potential mechanisms were studied in this set of experiments. Further investigations regarding the mechanism of cardioprotection induced by sildenafil suggested that protein kinase C is a crucial step in cardioprotection induced by sildenafil.46 In these experiments, chelerythrine, an inhibitor of protein kinase C, abolished infarct size reduction induced by sildenafil after 30 min of ischaemia and 3 h of reperfusion. In isolated mouse ventricular myocytes, sildenafil reduced necrosis and apoptosis after simulated ischaemia (replacing the cell medium with a modified ‘ischaemic buffer’, incubated at 1–2% O2).33 This protective effect of sildenafil was absent in inducible NO-synthase knock-out 207 mice and attenuated in endothelial NO-synthase knock-out mouse cardiomyocytes. These results point to NO signalling as an essential step in cardioprotection induced by PDE 5 inhibitors. Importantly, these isolated cell experiments were the first to demonstrate cardioprotective effects in ventricular cardiomyocytes completely independent from vascular responses or haemodynamic effects. Further experiments using isolated cardiomyocytes suggested that cardioprotection induced by sildenafil was dependent upon protein kinase G activated by cGMP, and involves phosphorylation of Erk 1/2 and glycogen synthase kinase 3ß.47 In Langendorffperfused mouse hearts, the same group investigated whether infarct size reduction induced by sildenafil administered prior to global ischaemia and reperfusion could be abolished by an Erk inhibitor. In this study, infarct size in control hearts (29.4 + 2.4%) was similar as in hearts after co-treatment with sildenafil and the Erk-inhibitor (31.8 + 4.4%), while sildenafil alone reduced infarct size to 15.9 + 3.0%, which provided strong evidence for direct involvement of Erk in sildenafil-induced cardioprotection in this model.48 These results regarding various signal pathways, in particular involvement of the NO system, may be important when considering potential clinical transferability of results. Diseases with altered NO signalling may result in different responses to sildenafil treatment.49 In dogs with alloxan- and streptozotocin-induced diabetes, sildenafil did not reduce infarct size after 60 min of ischaemia and 3 h of reperfusion, while pronounced attenuation of necrosis was found in non-diabetic dogs (16 + 2 vs. 31 + 2% of risk area).50 In contrast, another study, investigating a very low dose of sildenafil (0.06 mg/kg sildenafil) given 5 min prior to reperfusion after 30 min of coronary occlusion in wild-type mice, and inducible and endothelial NO-synthase knock-out mice demonstrated marked infarct size reduction in all three strains, which may suggest that additional mechanisms are involved in the cardioprotective effects of sildenafil.51 The mentioned studies highlight the need for detailed characterization of the experimental protocol, when investigating cardioprotective effects induced by phosphodiesterase 5 inhibitors. Concomitant diseases, such as diabetes or heart failure, might significantly alter the observed effects. 3.1.2 Cardiac pre- and afterload Administration of sildenafil reduced arterial blood pressure in most of the studies presented above. Depending on the velocity of intravenous application, a marked transient blood pressure drop may occur. For example, in the study by Ockaili et al.,39 0.7 mg/kg sildenafil reduced systolic, diastolic, and mean arterial blood pressure by 24.5, 47.3, and 38.8% respectively. Haemodynamics returned to nearly baseline levels within 5 min thereafter. In another study, when sildenafil was given slowly over 5 min, no acute blood pressure drop occurred, but blood pressure was reduced by 217 to 219 mmHg over 30 min.42 Regional myocardial blood flow was neither significantly increased nor reduced by sildenafil in this study, both in the ischaemic myocardial region and the non-ischaemic tissue. Taking reduction of blood pressure by sildenafil into account, PDE 5 inhibition significantly reduced specific coronary vascular resistance within the risk area, meaning that myocardial perfusion was not compromised in this experimental setting despite reducing coronary perfusion pressure, due 208 to coronary vasodilatation. Therefore, the reduction of arterial blood pressure, as an important determinant of cardiac afterload, may also contribute to cardioprotective effects during ischaemia, as it may reduce left ventricular work-load and energy expenditures during ischaemia without altering coronary blood supply.45 In addition, transient reduction of arterial blood pressure prior to coronary artery occlusion could serve as a preconditioning stimulus, similar to brief, non-lethal coronary artery occlusions, used to induce ischaemic pre-conditioning. Accordingly, in various experimental settings, protective effects by PDE 5 inhibitors may involve different mechanisms also including inadvertent pre-conditioning. Furthermore, PDE 5 inhibitors were shown to attenuate inotropic responses to catecholaminergic agents in several models.32,34,36,37,52 Therefore, reduction of cardiac inotropy during ischaemia should also be considered as a potential mechanism of infarct size reduction. Functional effects of PDE 5 inhibitors may not only be influenced by the degree of pre-activation of the NO-guanylyl cyclase pathway, but also by the degree of activation of the catecholaminergic system. Implicitly, this could mean that the depth of anaesthesia in experimental conditions could modify functional effects of sildenafil. It is important to keep in mind that PDE 5 inhibitors, at least in theory, could also increase cardiac contractility: as a mechanism of cross-talk between cGMP and cAMP, cGMP at high concentrations is known to partially inhibit PDE 3. This could in turn result in increased cAMP concentrations.53,54 Therefore, PDE 5 inhibition in cardiomyocytes and subsequently increasing cGMP levels could lead to secondary increase in cAMP-levels that may result in enhanced cardiac contractility. However, it is not clear whether this is of any relevance in clinical circumstances. As vasodilating effects of PDE 5 inhibitors occur both in the arterial, pulmonary, and venous system, cardiac preload might also be reduced under the influence of PDE 5 inhibitors. In a rabbit model, sildenafil during coronary occlusion resulted in normalization of end-diastolic left ventricular pressures, yet another potential cardioprotective mechanism. Similarly, sildenafil reduced left ventricular end-diastolic pressure without coronary occlusion.42 3.2 PDE 5 inhibitors at reperfusion Potential cardioprotective effects of vardenafil and sildenafil were also studied when given at reperfusion. Parallel to the phenomenon of ischaemic pre-conditioning and to pharmacological interventions mimicking ischaemic preconditioning, pharmacological interventions initiated at reperfusion may be compared with the experimental protocol of post-conditioning, where brief episodes of coronary re-occlusion within the very early phase of reperfusion are performed in an intention to reduce myocardial reperfusion injury.55 As the majority of cardiovascular events in the clinical arena are unpredictable, recent research has focused on interventions effective in reducing infarct size when initiated at reperfusion, which might be more realistic when transferring it to the clinical realm. In an open-chest rabbit model, infarct size after 30 min of coronary occlusion and 3 h of reperfusion was significantly reduced when sildenafil or vardenafil was started 5 min prior to reperfusion as an intravenous infusion and continued T. Reffelmann and R.A. Kloner for the first hour of reperfusion (19.2 + 1.3% with sildenafil, 17.0 + 2.0 with vardenafil vs. 33.8 + 1.7% of the risk area in controls).56 Cardioprotective effects were abolished by 5-hydroxydecanoate, again suggesting a mechanism involving opening of mitochondrial KATP-channels. Nitroglycerine, given at reperfusion, did not reduce infarct size in this set of experiments. The authors speculated that ‘rapid up-regulation of cGMP by PDE 5 inhibition rather than increasing its synthesis through NO’ is an effective approach to limit reperfusion injury in this model. A presumably more swift increase in intracellular cGMP after PDE 5 inhibition in comparison with guanylyl cyclase activation by nitroglycerine was discussed to be the pathophysiological basis for the observed differences. Additional direct effects of NO, other than increasing intracellular cGMP, might be an alternative explanation. With regard to the downstream cascade of signalling, they also discussed whether cGMP activation of the RISK-pathway via phosphatidyl-inositol-3 hydroxy-kinase-Akt and p44/p42 extracellular signal regulated kinases Erk 1/2 could be involved in mediating the observed cardioprotective effects, although there were no further data to support this hypothesis in this publication. Another study, investigating the effect of different doses of vardenafil, administered during 120 min of reperfusion after 30 min of ischaemia in isolated rat hearts, demonstrated infarct size reduction with 10 mM, but not with higher doses of vardenafil.57 Specific inhibitors of guanylyl cyclase and of protein kinase G prevented infarct size reduction in this study, suggesting that both guanylyl cyclase and protein kinase G are required for mediating vardenafil-induced cardioprotection.58 The authors also demonstrated restoration of the mitochondrial membrane potential under the influence of vardenafil despite challenge with calcimycin (calcium ionophore to induce mitochondrial permeability transition pores).57 This might indicate that vardenafil could exhibit its cardioprotective properties via inhibition of mitochondrial permeability transition pore formation which is regarded to be crucial for the determination of final infarct size under various cardioprotective interventions. These studies support other experimental work, in which various substances administered at reperfusion were shown to limit infarct size, presumably by reducing reperfusion injury, a potential attractive mechanism as an adjunct to reperfusion therapy for acute myocardial infarction in the clinical arena. 3.3 Cardioprotection late after application of PDE 5 inhibitors A substantial number of scientific publications on ischaemic pre-conditioning described a second, delayed phase of protection, a term used for infarct size reduction and attenuation of myocardial stunning even more than 24 h after a short period of sublethal ischaemia (the ‘pre-conditioning’ stimulus). Salloum et al.59 investigated whether similar effects late after exposure to sildenafil could be demonstrated. In these experiments, mice received an intraperitoneal injection of 0.7 mg/kg sildenafil or placebo 24 h prior to excision of the hearts. Immediately, the excised hearts were mounted on a Langendorff apparatus, and then subjected to 20 min of ischaemia followed by 30 min of reperfusion. Infarct size reduction by sildenafil was striking in this Phosphodiesterase 5 inhibitors study (6.9 + 1.2 vs. 27.6 + 3.3%). Furthermore, intraperitoneal injection of sildenafil was followed by a transient increase in inducible and endothelial NO synthase in the hearts between 45 and 120 min (mRNA level) and after 24 h (protein level). Inhibition of inducible NO synthase abolished cardioprotection by sildenafil. The involvement of increased expression of inducible NO synthase in the delayed cardioprotective response of sildenafil parallels other studies on delayed cardioprotection by ischaemic stimuli.60 A parallel set of experiments with a nearly identical protocol demonstrated that sildenafil-induced delayed cardioprotection could be blocked by inhibitors of the mitochondrial ATP-sensitive as well as the mitochondrial Caþþ activated potassium channel.59 In other experiments, using 30 min of ischaemia followed by 1 h of reperfusion, infarct size reduction by sildenafil was abolished both in adenosine-1-receptor knock-out mice and under the influence of a specific antagonist of the adenosine 1 receptor,61 suggesting involvement of the adenosine-1 receptor in signalling of sildenafil-induced delayed protection. In a Langendorff model, involvement of the survival kinase Erk in late protective effects of sildenafil, similar to early cardioprotective effects (see above), could also be demonstrated.48 Interestingly, delayed cardioprotective effects may be amplified by oral pre-treatment with a statin, as suggested in a study investigating the effects of 30 min ischaemia and 240 min of reperfusion in rats.62 4. Effects of phosphodiesterase 5 inhibitors on heart failure and the development of cardiac hypertrophy In a chronic murine model of myocardial infarction-induced heart failure, the effect of sildenafil treatment over 4 weeks was tested.63 After coronary artery ligation, 0.71 mg/kg sildenafil was given (intraperitoneal injection bid) or placebo. Infarct size, measured 24 h post-infarction, was significantly smaller in sildenafil-treated animals (40.0 + 4.6 vs. 69.6 + 4.1%), a protective effect that could be blocked by an inhibitor of NO synthase. Western blot analysis demonstrated a significant increase of inducible and endothelial NO synthase after 24 h. Apoptosis was significantly reduced by sildenafil. Furthermore, parameters of left ventricular remodelling, such as end-diastolic left ventricular dimensions, fractional shortening, and cardiac hypertrophy, were favourably influenced by sildenafil treatment which resulted in a pronounced difference in survival over 4 weeks (36 vs. 93%). In a model of doxorubicin-induced cardiotoxicity in mice, sildenafil or saline was administered prior to doxorubicin treatment (total dose of doxorubicin 15 mg/kg).64 Sildenafil (0.7 mg/kg intraperitoneally prior to doxorubicin) appeared to protect the hearts from doxorubicin toxicity, namely attenuating doxorubicin-induced cardiomyocyte apoptosis, and improving left ventricular developed pressure, as assessed by a Langendorff preparation (for example after 8 weeks: 92 + 1 mmHg for co-treatment with sildenafil and doxorubicin vs. 62 + 3 mmHg for co-treatment with saline and doxorubicin). Importantly, in these experiments, an inhibitor of NO synthase and an inhibitor of the mitochondrial ATP-sensitive potassium channel abrogated sildenafil-induced protection. In the tachycardia-induced heart failure model in dogs, described by Senzaki et al.,32 209 PDE 5 inhibition had little effect on dobutamine-induced increase in contractility. In animals without heart failure, however, PDE 5 inhibition markedly blunted inotropic responses to dobutamine. Similarly, PDE 5 expression was reduced in failing hearts and the co-localization of PDE 5 with z-bands of the sarcomere, which appeared to be characteristic in normal hearts, was not preserved in the failing hearts. The results suggest different effects of PDE 5 inhibition when heart failure has already developed, a fact that could be important when transferring the results to the clinical arena. A study by Chen et al.65 also suggested altered PDE 5 expression in the coronary vasculature in models of heart failure. PDE 5 inhibition was also tested in models of hypertrophic heart failure and ventricular remodelling, induced by increasing after-load. Sildenafil was shown to prevent or even reverse cardiac hypertrophy and exhibit beneficial effects on parameters of ventricular remodelling.66,67 In a mouse model of transverse aortic constriction, sildenafil was demonstrated to arrest progression of hypertrophy, to increase protein kinase G activity, and to enhance calcium transients at a time point, when hypertrophy had already developed.67 In this study, an increase in cardiac left ventricular mass of þ135% and increase in left ventricular enddiastolic dimensions of þ10% occurred within three weeks of aortic constriction. While left ventricular mass further increased in mice treated with placebo, starting sildenafil treatment after the first 3 weeks almost completely arrested the development of hypertrophy at this level.67 In a similar model, PDE 5 inhibition attenuated the development of left ventricular hypertrophy and left ventricular dilation.68 These potential beneficial effects were not exclusively dependent on the calcineurin pathway, which is known to be a target of protein kinase G. Hypertrophy and depressed heart function in mice lacking a subunit of calcineurin was favourably influenced by sildenafil in these experiments. Alternative pathways, such as the phosphoinositol-3 kinase Akt and Erk 1/2 signalling pathways, may also contribute to the cGMP signalling of hypertrophic responses.66 Other chronic animal models investigated whether cardiac morphological and functional changes induced by treatment with inhibitors of the NO pathway could be attenuated by sildenafil.69 After 8 weeks of treatment with L-nitro-arginine-methyl-ester, an inhibitor of NO synthesis, in rats, arterial hypertension developed, which was accompanied by a reduction of cardiac output and morphological changes of the left ventricle. Co-treatment with sildenafil significantly reduced myocardial lesions and attenuated the reduction in cardiac output compared with NO inhibition alone in these experiments. However, from most of the protocols, it is not absolutely clear which differential effects of PDE 5 inhibition are crucial: its effect on the cardiomyocyte, vasodilatatory effects on the systemic vasculature, or both. It is of particular interest that protective effects in these chronic experiments were closely related to the NO pathway. In humans, for example, treatment with tadalafil for 4 weeks resulted in significantly improved endotheliumdependent vasodilation, assessed as flow-mediated dilation of the brachial artery.70 As endothelium-dependent vasodilation is regarded as a crucial step in the pathophysiology of congestive heart failure, one might suggest that PDE 5 inhibition could also be beneficial via this mechanism.71 210 Some clinical studies examined effects of PDE 5 inhibition in heart failure.72,73 Hirate et al.,72 for example, found increased cardiac indices and decreased peripheral resistance 60 min after 50 mg sildenafil in patients with systolic heart failure. Therefore, PDE 5 inhibition may be an attractive pharmacological principle in heart failure patients with direct effect on the myocardium and also additive vascular effects. 5. Other cardiac effects of phosphodiesterase 5 inhibitors Other cardiac effects of PDE 5 inhibitors have been less well investigated. High doses of sildenafil were reported to influence membrane ion currents. The rapid component of the inward rectifier potassium channel was blocked by high concentrations of sildenafil in a study by Geelen et al.8 However, it is not clear whether this is of any relevance in clinical circumstances. In an animal model of right ventricular pacing, the threshold for arrhythmia under co-treatment with sildenafil and NO donors was lowered.9 According to the prescribing information, vardenafil slightly prolongs electrocardiographic QTc (heart rate corrected QT interval).74 This does not seem to be the case for sildenafil and tadalafil. In patients with heart failure, no promotion of arrhythmia was reported; however, a slight increase in sympathetic heart rate modulation occurred in one study.75 Sympathetic activation by a central nervous system-dependent effect was described in patients in another study, but its clinical relevance is not clear.76 6. Perspective and summary The foregoing discussion depicts a complex puzzle of studies, mainly from animal research, suggesting cardioprotective effects of phosphodiesterase 5 inhibitors including different models and mechanisms. Some of the observed effects resemble the basic mechanisms of ischaemic preconditioning, mimicking both acute and delayed effects. Other effects may be due to action on systemic and cardiac haemodynamics. As several investigations reported controversial results, close attention to the specific experimental protocol should be paid when estimating potential clinical applications of PDE 5 inhibitors in cardiovascular disease. The following aspects appear to be of outstanding importance and should be clarified before the value of the observed effects can be estimated for clinical circumstances (compare Figure 1): First, some of the studies investigating infarct size reduction after PDE 5 inhibition reported a very narrow dose-effect relationship.40,41,57 As both theoretical concepts53,54 as well as the study by Das et al.40 suggested, the possibility of exacerbation of myocardial necrosis under certain experimental conditions, the exact dosing, being safe and effective, must be defined very accurately. Second, many of the observed effects appeared to be dependent on adequate functioning of the NO–guanylyl cyclase pathway. Therefore, conditions, which potentially could lead to altered NO signalling,49 such as diabetes mellitus50 or heart failure,32 must be separately investigated, also with respect to potentially unforeseen effects. Third, T. Reffelmann and R.A. Kloner pre-activation of the nitric oxide–guanylyl cyclase pathway may determine the functional and potential protective effects of PDE 5 inhibition. This should be carefully accounted for both in experimental and in clinical situation. Co-administration of NO donors and PDE 5 inhibitors, for example, is absolutely contra-indicated. Fourth, in experimental conditions of ischaemia and reperfusion, inadvertent pre-conditioning due to blood pressure lowering effects of PDE 5 inhibitors prior to occlusion should be differentiated from direct cardioprotective effects, also mimicking ischaemic pre-conditioning. Fifth, pre-activation of the ß-adrenergic pathway in cardiomyocytes also appears to determine functional and potentially protective effects of PDE 5 inhibition.32 In experimental animal research, this should be carefully standardized, including the depth of anaesthesia. Also in clinical circumstances, this modulatory effect should be accounted for, in particular as conditions such as congestive heart failure might significantly alter this interaction.32 Sixth, the compartmentalization of cGMP, its functional effects, and enzymatic control should be further investigated in order to understand to what extent effects of cGMP derived from particulate guanylyl cyclase, for example in response to natriuretic peptides, differs from cGMP derived from soluble guanylyl cyclase, and whether this transfers into different cardioprotective action. In summary, further research on cardioprotective effects should be consequently pursued as the discussed investigations provide promising results with the perspective of clinical therapeutic strategies in coronary artery disease, as an adjunct to reperfusion therapy for myocardial infarction or as an additional strategy to improve symptoms and presumably improve prognosis in congestive heart failure. Conflict of interest: R.A.K. is a speaker, consultant, and researcher for Pfizer, Lilly ICOS, and Bayer (T.R.: no conflict of interest). References 1. Reffelmann T, Kloner RA. Cardiovascular effects of phosphodiesterase 5 inhibitors. Curr Pharm Des 2006;12:3485–3494. 2. Kloner RA. Cardiovascular risk and sildenafil. Am J Cardiol 2000;86: 57F–61F. 3. Feenstra J, van Drie-Pierik RJ, Lacle CF, Stricker BH. Acute myocardial infarction associated with sildenafil. Lancet 1998;352:957–958. 4. Porter A, Mager A, Birnbaum Y, Strasberg B, Sclarovsky S, Rechavia E. Acute myocardial infarction following sildenafil citrate (Viagra) intake in a nitrate-free patient. Clin Cardiol 1999;22:762–763. 5. Przyklenk K, Kloner RA. Sildenafil citrate (Viagra) does not exacerbate myocardial ischemia in canine models of coronary artery stenosis. J Am Coll Cardiol 2001;37:286–292. 6. Traverse JH, Chean YJ, Du R, Bache RJ. Cyclic nucleotide phosphodiesterase type 5 activity limits blood flow to hypoperfused myocardium during exercise. Circulation 2000;102:2997–3002. 7. Herrmann HC, Chang G, Klugherz B, Mahoney PD. Hemodynamic effects of Sildenafil in men with severe coronary artery disease. N Engl J Med 2000;342:1622–1626. 8. Geelen P, Drolet B, Rail J, Berube J, Daleau P, Rousseau G et al. Sildenafil citrate (Viagra) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. Circulation 2000;102:275–277. 9. Swissa M, Ohara T, Lee MH, Kaul S, Shah PK, Hayashi H et al. Sildenafilnitric oxide donor combination promotes ventricular tachyarrhythmia in the swine right ventricle. Am J Physiol Heart Circ Physiol 2002;282: H1787–H1792. 10. Wysowski DK, Farinas E, Swartz L. Comparison of reported and expected deaths in sildenafil (Viagra) users. Am J Cardiol 2002;89:1331–1334. Phosphodiesterase 5 inhibitors 11. Kloner RA. Cardiovascular effects of 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation 2004; 110:3149–3155. 12. Kloner RA, Mohan P, Segerson T. Cardiovascular safety of vardenafil in patients receiving antihypertensive medications: a post-hoc analysis of five placebo-controlled trials. J Am Coll Cardiol 2003;41(Suppl. A): 276A–277A. 13. Kloner RA, Jackson G, Hutter AM, Mittleman MA, Chan M, Warner MR et al. Cardiovascular safety update of Tadalafil: retrospective analysis of data from placebo-controlled and open-label clinical trials of Tadalafil with as needed, three times-per-week or once-a-day dosing. Am J Cardiol 2006;97:1778–1784. 14. Reffelmann T, Kloner RA. Pharmacotherapy of erectile dysfunction: focus on cardiovascular safety. Expert Opin Drug Saf 2005;4:531–540. 15. Reffelmann T, Kieback A, Kloner RA. The cardiovascular safety of tadalafil. Expert Opin Drug Saf 2008;7:43–52. 16. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinmann KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachussetts Male Aging Study. J Urol 2000;163:460–463. 17. Gross GJ. Sildenafil and endothelial dysfunction in humans. Circulation 2005;111:721–723. 18. Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient. Endothelial dysfunction is the common denominator. Heart 2003;89:251–253. 19. Kaiser DR, Billups K, Mason C, Wetterling R, Lungberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol 2004;43:179–184. 20. Maas R, Schwedhelm E, Albsmeier J, Borger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med 2002;7:213–225. 21. Reffelmann T, Kloner RA. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation 2003;108:239–244. 22. Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S et al. Guanylyl cyclases and signalling by cyclic GMP. Pharmacol Rev 2000;52: 375–414. 23. Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med 2005;230:242–250. 24. Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA et al. Compartmentalization of cardiac ß-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation 2007;115:2159–2167. 25. Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effect of Sildenafil on tissue cyclic nucleotides, platelet function, and the contractile response of trabeculae carneae and aortic rings. Am J Cardiol 1999;83:3C–12C. 26. Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of Sildenafil citrate. Am J Cardiol 1999;83:35C–44C. 27. Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil. Am J Cardiol 2003;92(Suppl.):37M–46M. 28. Ghofrani HA, Voswinckel R, Reichenberger R, Olschewski H, Haredza P, Karadas P et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2004;44:1488–1496. 29. Steiner MK, Preston IR, Klinger JR, Hill NA. Pulmonary hypertension: inhaled nitric oxide, sildenafil and natriuretic peptides. Curr Opin Pharmacol 2005;5:245–250. 30. Fox KM, Thadani U, Ma PT, Nash SD, Keating Z, Czomiak MA et al. Sildenafil citrate does not reduce exercise tolerance in men with erectile dysfunction and chronic stable angina. Eur Heart J 2003;24:2206–2212. 31. Halcox JPJ, Nour KRA, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002;40:1232–1240. 32. Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates ß-adrenergic signalling in vivo and is down-regulated in heart failure. FASEB J 2001;15:1718–1726. 33. Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: essential role of nitric oxide signalling. J Biol Chem 2005;280:12944–12955. 34. Loughney K, Hill TR, Florio VA, Uher L, Rosman GJ, Wolda SL et al. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 30 ,50 -cyclic nucleotide phosphodiesterase. Gene 1998;216:139–147. 211 35. Stief CG, Ückert S, Becker AJ, Harringer W, Truss MC, Forssmann WG et al. Effects of Sildenafil on cAMP and cGMP levels in isolated human cavernous and cardiac tissue. Urology 2000;55:146–150. 36. Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 2005;96: 100–109. 37. Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits ß-adrenergic-stimulated cardiac contractility in humans. Circulation 2005;112:2642–2649. 38. Castro LRV, Verde I, Cooper DMF, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac monocytes. Circulation 2006;113:2221–2228. 39. Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 2002;283: H1263–H1269. 40. Das S, Maulik N, Das DK, Kadowitz RJ, Bivalacqua TJ. Cardioprotection with sildenafil, a selective inhibitor of cyclic 30 ,50 -monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res 2002;28:213–219. 41. du Toit EF, Rossow E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther 2005;19:23–31. 42. Reffelmann T, Kloner RA. Effects of sildenafil on myocardial infarct size, microvascular function, and acute ischemic left ventricular dilation. Cardiovasc Res 2003;59:441–449. 43. Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP-channels in rabbits. J Mol Cell Cardiol 2006;40:405–411. 44. Kukreja RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A et al. Cardioprotection with phosphodiesterse-5 inhibition—a novel preconditioning strategy. J Mol Cell Cardiol 2004;36:165–173. 45. Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res 2007;19: 55–61. 46. Das A, Ockaili R, Salloum F, Hawkins J, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 2004;286:H1455–H1460. 47. Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanisms of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 2008;283:29572–29585. 48. Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemiareperfusion injury in mice. Am J Physiol Heart Circ Physiol 2009;296: H1236–H1243. 49. Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. Sustained soluble guanylate cyclase stimulation offsets nitric oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther 2008;326:380–387. 50. Jamnicki-Abgegg M, Weihrauch D, Chiari PC, Krolikowski JG, Pagel PS, Warltier DC et al. Diabetes abolishes sildenafil-induced cGMP-dependent protein kinase-I expression and cardioprotection. J Cardiovasc Pharmacol 2007;50:670–676. 51. Elrod JW, Greer JM, Lefer DJ. Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol 2007;292:H342–H347. 52. Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 2007;101:1084–1095. 53. Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res 1999;84:1020–1031. 54. Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphdiesterases and implications for cardiac pathophysiology. Circ Res 2007;100:1569–1578. 55. Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res 2006;70:200–211. 56. Salloum FN, Takenoshita Y, Ockaili RA, Daoud V, Chou E, Yoshida K et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondiral KATP channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 2007;42: 453–458. 57. Maas O, Donat U, Frenzel M, Rütz T, Kroemer HK, Felix SB et al. Vardenafil protects isolated rat hearts at reperfusion dependent on GC and PKG. Br J Pharmacol 2008;154:25–31. 212 58. Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase G in myocardial ischemia and reperfusion: opportunities and obstacles for survival signalling. Br J Pharmacol 2007;152:855–869. 59. Salloum F, Ying C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 2003;92:595–597. 60. Wang Y, Guo Y, Zahng SX, Wu W-J, Wang J, Bao W et al. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 2002;34:5–15. 61. Salloum FN, Das A, Thomas CS, Yin C, Kukreja RC. Adenosine A(1) receptor mediates delayed cardioprotective effect of sildenafil in mouse. J Mol Cell Cardiol 2007;43:545–551. 62. Rosanio S, Ye Y, Atar S, Rahan AM, Freeberg SY, Huang MH et al. Enhanced cardioprotection against ischemia-reperfusion injury with combining sildenafil with low-dose atorvastatin. Cardiovasc Drugs Ther 2006;20:5–8. 63. Salloum FN, Abbated A, Das A, Houser JE, Mudrick CA, Qureshi IZ et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 2008; 294:H1398–H1406. 64. Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterse-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 2005;111:1601–1610. 65. Chen Y, Traverse JH, Hou M, Li Y, Bache RJ. Effect of PDE5 inhibition on coronary hemodynamics in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 2003;284:H1513–H1520. 66. Takimoto E, Champion HC, Belardi D, Ren S, Rodriguez ER, Bedia D et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005;11:214–222. 67. Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedia D, Gabrielson KL et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing T. Reffelmann and R.A. Kloner 68. 69. 70. 71. 72. 73. 74. 75. 76. advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 2009;53:207–215. Hsu S, Nagayama T, Koitabashi N, Zhang M, Zhou L, Bedjy D et al. Phosphodiesterase 5 inhibition blocks pressure overload-induced cardiac hypertrophy independent of the calcineurin pathway. Cardiovasc Res 2009;81:301–309. Ferreira-Melo SE, Yugar-Toledo JC, Coelho OR, De Luca IM, Tanus-Santos JE, Hyslop S et al. Sildenafil reduces cardiovascular remodeling associated with hypertensive cardiomyopathy in NOS inhibitor-treated rats. Eur J Pharmacol 2006;542:141–147. Rosano GMC, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol 2005;47:214–222. Jackson G. Phosphodiesterase type 5 inhibition in cardiovascular disease: experimental models and potential clinical applications. Eur Heart J 2002;4(Suppl. H):19–23. Hirate K, Adji A, Vlachopopoulos C, O’Rourke MF. Effect of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol 2005; 96:1436–1440. Bocchi EA, Guimarães C, Mocelin A, Bacal F, Belotti G, Ramires JF. Sildenafil effects on exercise, neurohumoral activation, and erectile dysfunction in congestive heart failure: a double-blind, placebocontrolled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation 2002;106:1097–1103. Product monograph for Levitra (vardenafil HCL), Bayer Health Care, Glaxo SmithKline, 2003. Piccirillo G, Nocco M, Lionetti M, Moise A, Naso C, Marigliano V et al. Effects of sildenafil citrate (Viagra) on cardiac repolarization and on autonomic control in subjects with chronic heart failure. Am Heart J 2002;143:703–710. Phillips BG, Kato M, Pesek CA, Winnicki M, Nrkiewicz K, Davison D. Sympathetic activation by sildenafil. Circulation 2000;102:3068–3073.