* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Acid-base balance
Survey
Document related concepts
Transcript
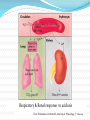
Acid Base Balance Dr. M. A. Maleque Molla, FRCP(Ed), FRCPCH October 22, 2014 Pre review Q 1 What is the normal range for arterial blood pH? A. 7.38 – 7.46 7.40 – 7.52 C. 7.35 – 7.45 D. 7.45-7.55 B. Answer C Q2 What 2 extracellular substances work together to regulate pH? A. Sodium bicarbonate & carbonic acid B. Carbonic acid & bicarbonate C. Acetic acid & carbonic acid D. Hydrochloric acid & bicarbonate Answer-B Q3 Defining an acid & a base, based on the choices below, which statement is true? Acids accept H+ ions & bases release H+ ions B. Acids release hydrogen (H+) ions & bases accept H+ ions C. Acids accept H+ ions & bases accept H+ ions D. Acid release H+ & Base Release H+ A. Answer: B Q4 Buffering is a normal body mechanism that occurs rapidly in response to acid-base disturbances in order to prevent changes in what? A. B. C. D. HCO3 H2CO3 H+ CO2 Answer: C Acid Base Balance Terminology & Definitions: Acid - Any substance that can donate proton (H+) e.g. H2CO3, NH3, HCL, ● Base- Any substance that can accept proton e.g. HCO3,PO4, protein ● Acidemia- pH< normal range(7.35-7.45). i.e. H+ in the blood above normal range ● Alkalemia-pH>normal (7.35-7.45). i.e. H+ in the blood less than normal range ● An acidosis & alkalossis is a pathologic process that causes an increase or decrease in the hydrogen ion concentration, Terminology & Definitions pH-Negative log of the hydrogen ion concentration pH= pK + log([base]/[acid]) Represents the hydrogen concentration ● Neutral pH is 7 at temp 250 C, 6.8 at 370C (Water) ● Normal pH of body fluids: Arterial blood is 7.4 (7.35-7.45) Venous blood and interstitial fluid is 7.35 Intracellular fluid pH 7.0 Acidosis -Blood pH <7.35 Alkalosis- Blood pH > than 7.45 ● pH compatible with life 6.8-7.8 Terminology & Definitions Buffers are substances that attenuate the change in pH that occurs when acids or bases are added to the body. Physiologic buffers: are weak acid and or of a weak base and its salt: bicarbonate-carbonic acid buffering system, intracellular protein buffers, phosphate buffers in the bone What does Buffer do? Buffers protect the body against major changes in pH, by binding or releasing the hydrogen ions(H+) Acid-Base Balance Acid-base balance: a normal balance between production and excretion of acid or alkali by the body, resulting in a stable concentration of H+ in body fluids An adult normally produces 1-2 mEq/kg/24 hr of hydrogen ions. Children produce 2-3 mEq/kg/24 hr of hydrogen ions. The 3 principal sources of hydrogen ions: Dietary protein metabolism, Incomplete metabolism of carbohydrates and fat, losses of bicarbonate in the stool In order to maintain acid-base homeostasis, acid production must balance the neutralization or excretion. Why acid-base balance? Regulation of normal pH is necessary because; Cellular enzymes and other metabolic processes, function optimally at normal pH. Chronic derangements in acid-base status may interfere with normal growth and development Acute, severe changes in pH can be fatal. How acid-base balance regulates? Body strictly maintains pH in a range from 7.35-7.45 Control of normal acid-base balance depends on; Lungs( Respiratory system). 2. Kidneys, (Renal System). 3. Intracellular and extracellular buffers. 1. 1. Respiratory Control mechanisms on pH Works within minutes to control pH- maximal in 12-24 hours Major source of acid in the body is CO2 CO2 react with water produce 12,500 mEq of H+ daily CO2+ H2O = H2CO3= H+ + HCO3 Excess CO2 & H+ in the blood act directly on respiratory centers in the brain increase rate & depth of respiration H+ stimulates respiratory center ↑ the respirations eliminate CO2 In case of alkalosis(pH>7.45), respiratory center is inhibited and there is retention of CO2 2. Renal Control Mechanisms on pH Kidney takes several hours to days to act & restore pH to, or close to normal Kidneys handles around 4000 mEq of HCO3 daily Almost all HCO3 are absorbed in renal tubules Regulate pH through excreting acidic or alkaline urine by; Excreting excess H+ & regenerating or reabsorbing HCO3 Excreting acidic urine decreases acid in the ECF & excreting alkaline urine removes base Respiratory & Renal response to acidosis From Thibeodeau GA PattonKI, Anatomy & Physiology, 5th ed,2004 3. Intracellular and extracellular buffers Bicarbonate/carbonic acid buffer 2. Protein buffers 1. 3. Phosphate Buffer Blood Buffer system 1. Bicarbonate/carbonic acid buffer Most abundant buffer in ECF Function almost instantaneously Cells utilizing O2 & produces CO2 CO2 combines with water to form carbonic acid(H2CO3), which dissociates to form H+ and HCO-3 : H2O+CO2 H2CO3 H+ + HCO2- If acid added to the fluid, combines with HCO3 and dissociate to H2O & CO2 Buffer system (Cont..) 2. Protein buffers: Extracellular proteins, mostly albumin Intracellular proteins, including hemoglobin. Protein buffers both hydrogen ions(H+ )and carbon dioxide(CO2) Buffer system (Cont..) 3. Phosphate Buffer System Phosphate is an intracellular buffer & important buffer in the Urine Has a major role in the elimination of H+ via the kidney • Assists in the exchange of sodium for hydrogen • It participates in the following reaction HPO-24 + H+ H2PO – 4 Essential within the erythrocytes Disorder in acid base balance Disorder in acid base balance ● ACIDOSIS (pH<7.35) Metabolic acidosis Respiratory acidosis Mixed acidosis(Combined ) ● ALKALOSIS(pH>7.45) Metabolic alkalosis Respiratory alkalosis DIAGNOSIS Clinical history & Examination Evaluation of an arterial blood gas sample can usually explain the patient's acid-base disturbance. Terminology used pH-Negative logarithm of hydrogen ion concentration PaCO2 -Partial pressure of carbon dioxide in arterial blood. PaO2- Partial pressure of oxygen in arterial blood HCO3- Bicarbonate concentration in the serum in mmol/l BE- calculated the quantity of Acid or Alkali required to return the plasma in-vitro to a normal pH under standard conditions Standard BE: is a calculation of the bicarbonate value if the blood were to be equilibrated with a PaCO2 of 5.3 kPa (40 mmHg ) How blood gas can be analyzed? Blood gas can be analyzed by automated machine using following types of blood samples: Arterial Blood Gas (ABG) Venous Blood Gas (VBG) Capillary Blood Gas (CBG) Normal values ABG VBG CBG pH 7.35-7.45 7.25-7.35 7.35 – 7.45 PCO2 (mmHg) 35-45 41-51 35 – 48 PO2 (mmHg) 80-100 35-40 80-100 HCO3 (mmol/L) 22-26 22-26 22 – 27 ±2 ±2 BE (mmol/L) ±2 Stepwise interpretation of blood gas Step I: Acidosis or Alkalosis 1. Look for pH Normal pH 7.35-7.45 ● pH < 7.35 Acidosis ● pH>7.45- Alkalosis Stepwise interpretation of blood gas Step 2: Respiratory or Metabolic 2. Look for pCO2 paCO2 > 45mmHg Respiratory Acidosis paCO2 < 35mmHg Respiratory Alkalosis 3. Look for HCO3 HCO3- < 22mEq/L Metabolic Acidosis HCO3- > 26mEq/L Metabolic Alkalosis Stepwise interpretation of blood gas Step 3: If respiratory acidosis ? Acute or Chronic Look for bicarbonate Normal slightly raised HCO3 – Acute High HCO3 -chronic Stepwise interpretation of blood gas Step 4: For metabolic acidosis Look for anion gap Nomal anion gap 12 ±4 mmol/l Anion gap > 16mmol/l- Anion gap metabolic acidosis Anion gap 12 ±4mmol/l =Normal or non-anion gap acidosis Anion gap Def: Calculated difference between anion & cation electrolytes Anion gap = (Na+ +K+ ) - (Cl- + HCO3-), Normal: 12 mmol/l • Nomal anion gap 12 ±4 mmol/l Anion gap metabolic acidosis (anion gap > 16): Uremia DKA(diabetic hyperglycemia) Alcohol, methanol, ethylene glycol, paraldehyde, salicylates poisoning Lactic acidosis (sepsis, left ventricular failure) Normal or non-anion gap acidosis (anion gap 12 ± 4) GI loss of HCO3 (diarrhea) Renal loss of HCO3 Renal tubular acidosis Compensation for respiratory alkalosis Ureteral diversion Other causes: HCl or NH4Cl infusion, Cl gas inhalation, Stepwise interpretation of blood gas Step 5. Determine whether other metabolic disturbances co-exist with an anion gap acidosis Formula; Corrected HCO3- = measured HCO3- + (anion gap - 12) If the corrected HCO3- is greater than 24, a metabolic alkalosis co-exists. If the corrected HCO3- is less than 24 then a non-gap acidosis co-exists. Stepwise interpretation of blood gas Step 6: Look for compensatory response Compensatory responses: The body’s attempt to return the acid/base status to normal Immediate buffering by ECF HCO3 Respiratory compensation: For each 1 mmol decrease in HCO3-a 1 mmHg drop of PaC02 Tissue phase: Entry of H+ into cells accounts for ~60% of rapid (~2 h) buffering of poorly permeable acids (HCl or H2SO4). Renal compensation Compensatory responses and their mechanisms. Primary disorder Primary Chemical change Compensatory response Compensatory Mechanism Metabolic Acidosis ↓ HCO3- ↓ PCO2 Hyperventilation Metabolic Alkalosis ↑ HCO3- ↑PCO2 Hypoventilation Respiratory Acidosis ↑PCO2 ↑HCO3- Acute Intracellular Buffering Chronic Renal Generation HCO3- Respiratory Alkalosis ↓ PCO2 ↓HCO3- Acute Intracellular Buffering Chronic Renal Generation HCO3- ABG Interpretation 1 pH = 7.202 PaCO2 = 19.8 PaO2 = 86.6 HCO3- = 7.4 BE = -18. Sat = 91.5 Hb = 12 Na+ = 153 K+ = 3.4 Cl- =123 •Metabolic acidosis •? Anion gap Anion gap=(153+3.4)-(123-7.4)= 26 ∆ Anion gap metabolic acidosis ABG Interpretation 2 ABG pH 7.31 pCO2 33 mmHg HCO3 16 mmol/l PO2 93 mmHg Na+ 134, K+ 2.9, Cl- 108, BUN 31, Cr 1.5. ∆ Metabolic Acidosis ?Anion gap (134+2.9)-(108+16)=12.9 ∆ Non anion gap metabolic acidosis Metabolic acidosis Def: pH<7.35 due ↑ H+ concentration Cause: ● Exogenous source ● Endogenous ↑production; DKA, organic acidemia, lactic acidosis ● Inadequate excretion; Renal failure ● Excessive loss of HCO3; GE Metabolic acidosis Consequence Buffered by ● ECF - HCO3 ● ICF – Hb, Po4, bone ● Respiratory compensation: ↑RR ↓ PCO2 ● Renal compensation: ↑ Ammonia production + H+ excretion ↑HCO3 reabsorption Metabolic acidosis Clinical features ● Hyperventilation ● Decrease cardiac function ● Hypotension ● Pulmonary oedema ● Hypoxia Metabolic acidosis -Diagnosis History & Physical examination Lab: BUN, serum creatinine, serum glucose, urinalysis, and serum electrolytes Plasma anion gap: Those with normal anion gap e.g. GE, RTA Those with increased anion gap. ↑endogenous proudction of acid e.g. DKA, Organic acidemia, Lactic acidosis Exogenous Acid e.g. Ethylene glycol, Methanol, Salicylate, Paraldehyde Acid base status : pH <7.35, ↓ PCO2 ↓ HCO3 ABG Interpretation 3 pH PCO2 PO2 HCO3 BE 7.29 64.3 mmHg 84.6 mmHg 24.2 mmol/l -2 mmol/l Acute Respiratory acidosis Respiratory acidosis Inadequate elimination of CO2 due to respiratory failure Causes: Obstruction Neuromuscular disease Sedative over dose Compensation mainly by renal reabsorption of HCO3 Acid base status: pH <7.35, ↑PCO2, Normal or ↑HCO3 Respiratory acidosis -Management Treatment: with an acute respiratory acidosis are hypoxic and need oxygen. Mechanical ventilation in some children Indication for mechanical ventilation Severe Respiratory acidosis PCO2 >75 mmHg & pH<7.2 Concomitant metabolic acidosis, slowly responsive underlying disease, Hypoxia that responds poorly to oxygen, If the patient is exhausted and respiratory arrest seems likely ABG Interpretation 4 pH PCo2 PO2 SAT HCo3 7.489 24.9 mmHg 72.4 mmHg 96.4% 21.6 mmol/l Respiratory alkalosis Respiratory alkalosis Excessive elimination of CO2 Cause ● Iatrogenic hyper ventilation ● Hyperventilation e.g early pneumonia, asthma ● Psychogenic ● Drugs like Salicylate poisoning Acid base status: pH >7.45, ↓ PCO2, ↓ HCO3 Respiratory alkalosis-Management Underlying cause should be treated If on mechanical ventilator, setting should be adjusted Psychogenic hyperventilation may benefit from reassurance benzodiazepines. Rebreathing into a paper bag. ABG Interpretation 5 pH = 7.490 PaCO2 = 47.0 PaO2 = 58.0 HCO3- = 34.8 BE = 10.2 Sat = 88.9 Hb = 18.3 ∆ Metabolic alkalosis Metabolic alkalosis Decrease acid below the normal range Causes: divided into 2 categories on the basis of urinary chloride level 1. Chloride responsive (Urinary chloride <15 mEq/l) Excessive loss of H+ e.g. vomiting, Nasogastric suction ● Diuretics (loop or thiazide) ● Decrease serum potassium, serum Cl-, ● Contraction of ECF ● Cystic fibrosis ● Chloride-losing diarrhea ● Post-hypercapnia 2. Chloride resistant Urinary chloride >20 ● Hyperaldosteronism ● Cushing syndrome, ● Bartter’s synd Metabolic alkalosis Clinical feature- muscle cramps, tetany, ● Respiratory compensation: ↓Respiratory rate, ↑ PCO2 ● Renal compensation: loss of HCO3 in urine Acid base status: pH >7.45 Normal or ↑ PCO2 ↑ HCO3 Low S Potassium Calcium specially Ionized Ca++ Management Depends on the severity of the alkalosis and the underlying etiology. In children with a mild metabolic alkalosis (HCO3− <32), treatment is often unnecessary Moderate or severe metabolic alkalosis need treatment Treat underlying cause e.g, if receiving diuretic add potassium sparing one Supplement of sodium chloride and potassium chloride to correct the volume deficit and the potassium deficit ABG Interpretation 6 pH Pco2 PO2 BE HCO3 SAT Na K Cloride 6.90 79.3 mmHg 25.2 mmHg -15.8 mmol/l 12 mmol/l 31.5% 136 mmol/l 4.1 mmol/l 120 mmol/l Combined respiratory & metabolic acidosis REFERENCES Bishop, M., Fody, E., & Schoeff, l. (2010). Clinical Chemistry: Techniques, principles, Correlations. Baltimore: Wolters Kluwer Lippincott Williams & Wilkins. Carreiro-Lewandowski, E. (2008). Blood Gas Analysis and Interpretation. Denver, Colorado: Colorado Association for Continuing Medical Laboratory Education, Inc Acid-Base Balance;Larry A. Greenbaum; Nelson textbook of Pediatrics, 19 ed