* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzymes
Survey
Document related concepts
Transcript
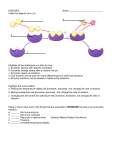
Enzymes An enzyme is a biological catalyst that accelerates a chemical reaction by lowering the energy needed to start the reaction without actually being permanently changed itself. So what is a catalyst ? A catalyst is a substance that increases the rate at which a reaction takes place. An enzyme is therefore an organic molecule that helps a reaction proceed without needing high amounts of energy. It causes reactions to happen much faster than they would if it wasn't present. Most enzymes are proteins and therefore have the same basic structure as proteins. Enzymes are actually globular proteins, they have an almost spherical shape. Many enzymes also contain or require nonprotein parts and only work properly when these parts are present. These can be lipids, sugars, metallic ions (e.g Ca2+) vitamins or substances derived from vitamins. These components are called cofactors. A cofactor is a substance that is essential for some enzymes to function efficiently. There are three types of cofactor: activators coenzymes prosthetic groups The first two cofactors are not permanently bound to the enzyme activators Many enzymes are not active in the sites where they are manufactured. They are purposely left incomplete. This is because they might otherwise catalyse reactions there that should not take place. This is particularly true of digestive enzymes and enzymes involved in blood clotting. If blood-clotting enzymes were always active, they would clot blood in the blood vessels all the time. Coenzymes are non-protein molecules. They are only present when the enzyme is busy catalysing a reaction and do not always bond to the enzyme. Many vitamins are either precursors to coenzymes (they will be turned into coenzymes) or are already coenzymes. An example of this is ascorbic acid (Vitamin C). All enzymes have a small area on their surface where the amino acid chains making up the enzyme form an indented area. This is where the catalytic action of the enzyme is (ie where the enzymes do their work). This is called the active site and is formed by the specific folding of the amino acid chains making up the protein Three main types of reaction occur in your body - catabolic reactions, anabolic reactions and reactions involving the turning of one substance into another (called conformational changes). Enzymes are able to catalyse all of these reactions. The substances that are undergoing the reaction are called the substrates. Active sites are places that the substrate molecules fit into. Most enzymes are very specific and able to accept only certain substrates. Enzymes are usually highly substrate specific. We need many thousands of different enzymes in order to catalyse the many thousands of reactions in the body. Some substances are able to "cheat" the enzyme into "thinking" that they are the correct substrates. The ability of an enzyme to work efficiently depends on the presence and position of amino acid residues in the active site and on the structure of the whole enzyme. The shape of the active site is also determined by the structure of the whole enzyme The shape of the active site is important in ensuring enzyme specificity. There are two theories concerning how the active site is able to accept only certain substrates: the lock and key mechanism the induced fit theory This theory states that the substrate (the key) fits exactly into the active site (the lock) of the enzyme. As there is usually only one key that fits a lock, there is usually only one substrate or group of substrates that fit the active site of a certain enzyme. This theory supposes that enzymes have active sites that do not change shape easily. So the way in which the substrate fits is purely a result of the shape of the substrate and the active site. Many enzymes have been found to have active sites that are not so rigid. The induced fit model is an extension of the previous model. It states that the active site only fits the shape of the substrate after the substrate has bound to the enzyme. Only the correct substrate can cause the active site to change shape to fit it. The way in which the active site changes its shape is through interactions with cofactors such as coenzymes, activators or even pieces of the substrate. These cause the breaking and reforming of certain hydrogen bonds in the enzyme structure and change the shape of the active site. Only the correct substrate will trigger the correct changes to the active site. When an enzyme is in solution with substrate molecules, the enzyme's active sites are able to accept substrate molecules only if it is the correct substrate. In anabolic reactions, the active site is formed specifically so that the substrates will be correctly aligned to bond together. In catabolic reactions and reactions involving internal molecular changes, the active site acts by breaking existing bonds and forming new ones. This is done through the action of the amino acid residues in the active site pulling the molecule apart. Substrates bound with the active site are called an enzyme-substrate complex. When the new product has formed, it is no longer attractive to the active site and leaves it. The new molecule is called the product. Enzymes can function both inside cells (intracellular) or outside cells (extracellular). For example, the enzymes that function in our digestive systems are manufactured in cells - but work extracellularly. Enzymes that act inside cells are responsible for catalysing the millions of reactions that occur in metabolic pathways such as glycolysis in the mitochondria The lysosomes contain many enzymes that are mainly responsible for destroying old cells Temperature – enzymes do not function at low or high temperatures PH- some have an optimum ph range at which they work. Enzyme Concentration Substrate Concentration An inhibitor is a substance that inhibits (restrains) the functioning of an enzyme. There are two main types: reversible inhibitors irreversible inhibitors Irreversible inhibitors are substances that bond to the enzyme covalently. They are not displaced by the substrate that usually binds to the enzyme (because the substrate binds with hydrogen bonds which are much weaker than covalent bonds). By bonding to the enzyme, either at the active site (so the substrate cannot bind there) or at any other part of the enzyme, the inhibitor may be able to change the conformation (shape) of the enzyme. It does this by either breaking hydrogen bonds or making others form in the wrong place. This changes the enzyme structure. These inhibitors are called irreversible because they do not easily leave the enzyme. The enzyme is no longer able to function. There are two types of reversible inhibitors: competitive non-competitive In both cases reversible inhibitors can be removed from the enzymes - but only under certain conditions. Competitive inhibitors are inhibitors that compete with substrates for the active site. They resemble the substrate in that they can fit into the active site, fooling the enzyme into thinking that they are substrates. They differ from the substrate in that they are unreactive. They therefore reduce the number of enzymes available to catalyse a reaction. If there is enough substrate available though, the chances of an enzyme attracting an inhibitor is smaller - most enzymes will still attract the correct substrates and a reaction can still occur. To help overcome the effect of a competitive inhibitor, the substrate concentration should be increased. The inhibitors usually leave the active site when the substrate concentration is high enough. These substances do not bind to the active site of an enzyme, but to other parts of the enzyme. In doing so, they may change the conformation of the active site and possibly inactivate it