* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Clinical Pharmacology - International Pain School
Pharmacokinetics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Dextropropoxyphene wikipedia , lookup
Pharmacogenomics wikipedia , lookup
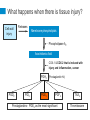
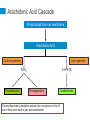
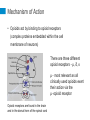
International Pain School Clinical Pharmacology of Analgesic Medications & Non-Phramacologcial Treatments type in your name type in the name of your institution Contents of this Lecture (1) Clinical Pharmacology of: • Paracetamol (Acetaminophen) • Non-steroidal anti-inflammatory drugs (NSAIDS) / COX-2 specific inhibitors • Opioids • Ketamine • Drugs used for neuropathic pain • Local Anaesthetic agents • Others - Steroids (2) Non-drug treatments Clinical Pharmacology includes • Mechanism of Action • • • • Absorption / Elimination of the drug Indication for use / dosage Adverse / side effects Any special precautions that should be taken Paracetamol • Paracetamol has been in use for more than a century • It has both analgesic and antipyretic action • However, the exact mechanism of its action is unclear Absorption / Elimination from the body • It is well tolerated when taken orally. • On oral administration it is absorbed from the intestine (70%), stomach and colon (30%) • The rate of absorption is rapid and depends on the dose Absorption / Elimination • The time taken to reach maximum plasma concentration (Tmax) is 15 - 30 minutes depends on the preparation • It is available as tablets (adults), suspension or syrup for children and suppositories • Tmax is 2 - 3 hours with suppositories • Bioavailability ranges from 60-90% Elimination • Paracetamol is metabolized in the liver and only 2 - 5% is excreted unchanged Indications and dosages • It is used as an analgesic drug for mild to moderate pain – E.g. Tooth ache / teething pain in children, backpain, joint and muscle pain, headache, dysmenorrhoea • Relief of fever in adults and children Dosage • Adults – Up to 1g oral / rectal, every 6 hours ( 4g should not be exceeded / day • Children – Oral / rectal 20 mg / kg – every 6 hours Side effects • Paracetamol is well tolerated and has no side effects at therapeutic doses • It has good haematological tolerability and does not alter haemostasis Caution • Since it is metabolized in the liver it must be used with caution / or omitted in the presence of liver impairment • In patients with renal impairment, the dose of paracetamol should be reduced • Do not exceed 4g/day in adults and 125 mg/ kg in children Adverse effects Hepatotoxicity with an overdose of paracetamol • This can occur when a patient does not get adequate relief with paracetamol and decides to take more than the prescribed dose of a maximum of 4g/day (8 - 10 g / day) • Intentional overdose (Paracetamol overdose / poisoning is the leading cause of acute liver failure in the US, UK and Australia) • Overdose causes acute liver failure, as the elimination pathways are saturated resulting in elevated levels of toxic metabolites Adverse Effects • N-acetylcysteine (NAC) is the antidote for paracetamol poisoning and it is most effective when administered within 8 - 10 hours after ingestion • Renal toxicity – Overdose can cause severe kidney necrosis • Allergic reactions are rare NSAIDs Non Steroidal Anti-inflammatory Drugs History of Aspirin • Salicylate from the bark of the willow tree and was used to treat fever and rheumatism for centuries • In the late 19th century, salicylic acid and later acetylsalicylic acid was synthesized and called aspirin. • Aspirin was widely used to treat fever and pain till the availability of other drugs with similar mechanisms of action. It continues to be used in many parts of the world Non-Steroidal Anti-inflammatory Drugs (NSAIDs) • They are diverse group of compounds which were later synthesized, with actions similar to that of aspirin and became known as NSAIDs • The mechanism of action of aspirin / NSAIDs was discovered in the 1960’s by Prof Vane, who was awarded a Nobel prize in Medicine in 1982 Non-steroidal Anti-inflammatory Drugs NSAIDs • NSAIDs are widely used to treat pain and inflammation • They act through inhibition of the two isoforms of the enzyme cyclooxygenase (COX) – i.e. COX-1 and COX-2 • NSAIDs that act on both the enzymes are known as non-selective NSAIDs (ns-NSAIDs) • NSAIDs which act predominantly on the COX-2 enzyme are known as specific COX-2 inhibitors (also referred to as Coxibs) The Two Isoforms of COX • COX-1 is a normal constituent in the body for homeostasis, such as in: – Gastric mucosa – gastric cytoprotection – Kidney – Sodium and water balance / renal perfusion – Platelets – for aggregation • COX-2 is induced in the presence of injury and inflammation • COX-2 is also a normal constituent in the many organs such as: Kidney, brain, endothelium, ovary and uterus What happens when there is tissue injury? Cell wall injury Releases Membrane phospholipids Phospholipase A2 Arachidonic Acid COX-1 & COX-2 that is induced with injury and inflammation, cancer PGH2 (Prostaglandin H2) PGD2 PGI2 PGE2 PGF2 Prostaglandins- PGE2 as the most significant TXA2 Thromboxane Arachidonic Acid Cascade Phospholipid from cell membrane Arachidonic Acid Cyclo-oxygenase Lipoxygenase PGH2 Thromboxane 5-HPETE Prostaglandins These inflammatory mediators activate the nociceptors on the Aδ and c fibres and result in pain and sensitization Leukotrienes Arachidonic Acid Cascade Phospholipid from cell membrane Arachidonic Acid NSAIDs / COX-2 inhibitors Cyclo-oxygenase Lipoxygenase PGH2 Thromboxane 5-HPETE Prostaglandins Reduce Prostaglandins and Thromboxane, resulting in reduced pain Leukotrienes ns-NSAIDs COX-2 specific inhibitors (= Coxibs) Acetylsalicylic acid (aspirin) Celecoxib • Tablet, suppository • Oral capsules Ibuprofen Etoricoxib • Tablet, suspension for children • Oral tablets Indomethacin Parecoxib • Tablet • parenteral Diclofenac • Oral tablet, suppositories, parenteral form available Mefenamic acid • Oral tablets Anti-Pyretics / NSAIDs on the WHO essential drug list : • Acetylsalicylic acid (Aspirin) – Tablet 100 mg to 500 mg – Suppository 50 mg to 150 mg • Ibuprofen > 3 months in age – Tablet 200 mg; 400 mg – Oral liquid: 200 mg / 5 ml • Paracetamol – Tablet 100 mg to 500 mg – Suppository: 100mg, 250 mg – Oral Liquid: 125 mg / 5 ml Absorption and Elimination • When administered orally, aspirin, ns-NSAIDs and Coxibs are well absorbed and reach therapeutic levels within 30 to 60 minutes. Indications • Both the ns-NSAIDs and Coxibs have the same efficacy in postoperative analgesia – Sole analgesia for day surgery – Along with opioids for major surgery • Musculo-skeletal pain – e.g. back pain, joints, muscle sprains etc. – Osteoarthritis – Rheumatoid arthritis • Not indicated for neuropathic pain Side effects / Adverse effects Gastrointestinal effects •The risk of erosions, ulcers and bleeding is higher with ns-NSAIDs compared to Coxibs. •This risk with ns-NSAIDs is also variable with some being less than others. • Risk is greater – In elderly patients – Those who are also taking aspirin •Risk can be reduced by adding a proton-pump inhibitor (e.g. omeprazole) to ns-NSAIDs. – H2 receptor blockers are not very effective. Renal effects • Both COX-1 & 2 are constituent enzymes in the kidney – Maintain renal perfusion and sodium/water balance • Both ns-NSAIDs and Coxibs can cause – Hypertension, odema – Decrease in creatinine clearance that may be significant in patients with impaired renal function or transient hypotension / hypovolaemia in the postoperative period Cardiovascular effects • Some studies have shown that there was a higher risk of thrombotic cardiovascular events (stroke, heart attack) when on Coxibs when compared to ns-NSAIDs such as naproxen • Other studies have shown that the cardiovascular events are similar • Nevertheless, current recommendations are that Coxibs should not be used in patients with active cardiovascular disease and a known thrombotic condition Effect on platelets • ns-NSAIDs are able to prevent platelet aggregation as platelets do not have COX-2. There is therefore a potential for bleeding with ns-NSAIDs • Coxibs do not prevent platelet aggregation • ns-NSAIDs should be used with caution in patients who are already on aspirin Others • Some ns-NSAIDs can precipitate asthma is aspirin sensitive asthmatic patients. • Coxibs are well tolerated by patients who have aspirin sensitive asthma Summary (cont.) NS-NSAIDs / Coxibs • Both drugs are effective in providing pain relief for moderate pain • The mechanism of action of both groups of drugs is by inhibiting the COX-2 enzyme that is induced with injury, inflammation and cancer • Gastrointestinal side effects are less with coxibs Summary NS-NSAIDs / Coxibs • Coxibs have no effect on platelet aggregation • Both drugs should be used with caution in patients with renal impairment and in the elderly • Coxibs should not be used in patients with active cardiovascular disease or known thrombotic effects • Coxibs can be given to patients with aspirin sensitive asthma • Both drugs should be used for the shortest period of time at the lowest dosage Opioids Opioids • Opium alkaloids derived from the opium poppy has been used for pain relief for centuries • Morphine was isolated by Sertuner in 1813 • The glass syringe was introduced in 1844 • Since then morphine has been the mainstay in the management of severe pain • The term “opioid” is referred to any drug, either natural, semi-synthetic or fully synthetic, which has actions similar to morphine Available Opioids Natural • Morphine • Codeine Semi-Synthetic • Hydromorphone • Oxycodone • Diacetylmorphine (heroin) • Naloxone (antagonist) Opioids on the WHO essential drug list • Morphine • Codeine • Tramadol Fully Synthetic • Pethidine (meperidine) • Tramadol • Nalbuphine • Methadone • Pentazocine • Fentanyl • Alfentanil • Sufentanil • Remifentanil Opioids can be classified as: • Strong opioids used for severe pain – Morphine, Oxycodone, Pethidine, Fentanyl • Weak Opioids used for moderate pain – Codeine, Tramadol The analgesic ladder for acute pain management Strong opioids Weak opioids Mechanism of Action • Opioids act by binding to opioid receptors (complex proteins embedded within the cell membrane of neurons) There are three different opioid receptors - µ, δ, κ µ - most relevant as all clinically used opioids exert their action via the µ -opioid receptor Opioid receptors are found in the brain and in the dorsal horn of the spinal cord Mechanism of Action • Opioids bind to opioid receptors • Activate intracellular signaling events • Leading to reduction in excitability of neurons and inhibition of pain signals • Resulting in reduction of pain perception Opioids can be administered via several routes Opioids produce potent analgesia when administered: • Systemically – oral, Intravenous, intramuscular, subcutaneous, transcutaneous, per rectal • Spinally – epidural, intrathecal, intraventricular Time to peak action and duration of action depends on the route and dose of the drug Morphine • Is the most widely used opioid for the control of severe pain • It can be given by all the routes that was described in the previous slide. • It is well absorbed when given orally and has a bio-availability of around 30-35%. • Bio-availability means the amount of drug that is available in the systemic circulation after an oral dose is given. Oral morphine Immediate release morphine • Aqueous / liquid morphine (usually prepared as 1-2 mg / ml) • Tablet morphine (10 mg) • Need to be given every four hours for continuous relief of severe pain Sustained Release (SR) Morphine Tablets • Morphine is released slowly over 12 hours • 10 mg, 30 mg, 60 mg • These tablets are given twice a day Parenteral morphine (10 mg / I ml ampoule) Intramuscular / subcutaneous morphine • Onset of Analgesia 15 - 20 min • Peak action 45 - 90 min • Duration of action 4 hours Intravenous route is chosen when rapid control of severe pain is desired. Metabolism The principle pathway of metabolism is conjugation with glucuronic acid in hepatic and extra-hepatic (kidney) sites • Morphine -3 and morphine -6 glucuronides that are excreted mainly by the kidneys • Morphine should be used with caution in patients with hepatic and renal impairment Codeine phosphate – Weak opioid • Oral tablet 15mg; 30 mg • Is well absorbed and there is no first pass metabolism in the liver • Codeine is metabolized to morphine; which accounts for its analgesic effect • 60 mg of codeine has an equi-analgesic effect of 650 mg aspirin • Has an anti-tussive effect and is often used in cough mixtures • Is available in combination with paracetamol • Cause minimal sedation, nausea, vomiting and constipation Tramadol – Weak opioid • This is also known as an “atypical opioid” • It has a dual mechanism of action: – weak opioid receptor binding properties – Inhibits the reuptake of serotonin and noradrenaline at the descending inhibitory pathway • It is available – Oral capsule (50 mg) – Injection – 50 mg / ml – in 2 ml ampoules • Due to its weak opioid activity it is not placed in the same schedule as the strong opioids such as morphine Tramadol • It is well absorbed when given orally • Time to effect is around 30 minutes and can last 5 - 6 hours • Sedation is minimal • Can cause nausea, vomiting, dizziness • Abuse potential is minimal • Is used as a weak opioid, however as it has a dual mechanism of action – its analgesic efficacy is superior to codeine – Maximum daily dose is 400 mg Metabolism • Tramadol is metabolized by the liver and excreted by the kidneys • Tramadol has an active metabolite (O-desmethyltramadol) – that is also excreted by the kidney • The daily dose should be reduced in the presence of chronic renal failure Opioid related side effects • Gastrointestinal – Nausea and vomiting – Constipation • Sedation • Respiratory depression in overdose • Pruritus • Cough suppression (anti-tussive) Opioid related side effects • On initiation of opioid therapy, patients frequently report acute side effects of sedation, dizziness, nausea and vomiting • After a few days these symptoms subside except for constipation • This is noted in patients with cancer pain Opioids and Tolerance Patients can develop tolerance when opioids are used for an extended period • E.g. cancer pain; intensive care units Tolerance is defined as reduction of the pharmacological effect of an opioid: • When the same dose produces a lesser effect • Increasing doses of drug is required to produce the same effect • The mechanisms of the development of tolerance are complex Physical Dependence and Addiction Physical dependence is a state of adaptation by the body with extended use of an opioid • It is manifested by withdrawal symptoms with abrupt cessation of the opioid, rapid dose reduction or administration of an opioid antagonist Addiction to opioids is drug seeking behaviour where the person is looking for opioids for its euphoric action rather than pain relief alone Ketamine – is on the WHO essential drug list • It is a phencyclidine derivative • It has been used to provide anaesthesia for many years – The side effect of hallucinations has limited its use as an anaesthetic agent – Ketamine causes an increase in blood pressure and heart rate, hence it is used as an induction agent when a patient is in shock • It has multiple mechanisms of action – The main mechanism of action is that of a noncompetitive antagonist at the NMDA receptors Ketamine • Routes of administration – Intravenous – Intramuscular / Subcutaneous (onset – 10 - 15 min) – Oral (bioavailability 20%) / sublingual (30%) / rectal (30%) • Metabolized by the liver – Metabolites are about 1/5th as potent as ketamine Ketamine has potent analgesic properties • Analgesia • Low dose ketamine – 0.1 - 0.5 mg / kg / hr can provide excellent analgesia – It can reduce opioid requirements in the postoperative period – It can be used for the management of neuropathic pain such as in patients with complex regional pain syndrome – As an adjunct for the relief of cancer pain, particularly for the neuropathic component Ketamine • 5mg / kg – IM can be used for painful dressing change in children • An anti-sialagogue should be added as it causes salivary secretions that can cause coughing / laryngospasm Side effects of ketamine • Dizziness • Hallucinations • Emergence delirium when larger doses are used – Benzodiazepines can reduce these side effects • Salivary secretions – Anti-sialagogues should be used with ketamine Ketamine and drug abuse • In recent years, ketamine has been known to be abused for its “euphoric” effects • Long term use can lead to cognitive impairment and memory loss Drugs for Neuropathic Pain Neuropathic pain • Is defined as pain that arises as a result of injury or disease of the somatosensory system • Neuropathic pain is not responsive to ns-NSAIDs. • Poorly responsive to Opioids Drugs used for treating neuropathic pain • Amitriptyline • Carbamazepine • Sodium valproate • Gabapentinoids (not on the WHO essential drug list) Amitriptyline • Is a tri-cyclic anti-depressant drug • Used more for the management of neuropathic pain than for symptoms of depression • Low dose amitryptyline is a first line drug for neuropathic pain • Mechanism of action – Inhibits the reuptake of noradrenaline and serotonin (thus increasing these two neurotransmitters) at the descending inhibitory pathway – The descending inhibitory tract influences the output of the neurons in the dorsal horn of the spinal cord Absorption / Elimination • Amitriptyline is well absorbed on oral administration – Bioavailability 30 - 60% – Effects last 2 - 12 hours • Metabolized in the liver (de-methylation) and excreted in the urine Adverse effects • Common ones – Dry mouth, disturbances of visual accommodation – Constipation and urinary retention – Light-headedness, drowsiness • Less common effects – Cardiac Arrhythmias It should be used cautiously in the elderly and in those with a history of cardiovascular disease Carbamazepine • Is an antiepileptic drug • It is currently the drug of choice for the management of pain in patients with trigeminal neuralgia • Mechanism of action – It blocks the frequency and use of the voltage-gated neuronal sodium channels – Limits repetitive firing action of action potentials – There is a proliferation of sodium channels when there is nerve injury – thus the efficacy of carbamazepine in patients with neuropathic pain Side effects • The most common side effects are neurotoxic and doserelated. They include: – Drowsiness, diplopia, headache, ataxia, nausea – Vomiting, dizziness – These side effects tend to occur within a week of initiation or dosage increase. • In chronic therapy, they typically are noticeable 3 - 4 hours after a dose (associated with peak serum concentrations) • Systemic effects – Abdominal pain, diarrhea, hyponatraemia in the elderly Serious side effects • Agranulocytosis and aplastic anaemia • Skin eruptions and life threatening Steven-Johnson’s syndrome • Blood tests should be done early in the course of therapy and patients should be asked to report easy bruising. Interaction with other drugs • Carbamazepine is an inducer of CYP450 in the liver – Efficacy of other drugs are reduced notably e.g. • Warfarin • Phenytoin • Valproic acid • Some drugs reduce the metabolism of carbamazepine and therefore increase its plasma level – Erythromycin – Cimetidine – Calcium channel blockers Local Anaesthetics (LA) • Lignocaine (short acting) – 0.5%, 1.0%, 2.0% solutions • Bupivacaine (long acting) – 0.5% • Both belong to the amide group of LA drugs • Mechanism of action – LA drugs act by producing a reversible block to the transmission of peripheral nerve impulses – i.e. they block membrane depolarization of all excitable tissue, in particular the nerves – This action is on the sodium channels of the peripheral nerves. LA drugs • Can provide both surgical anaesthesia and analgesia – Depends on the site of administration – Concentration of the drug used • Common routes of administration: – Local infiltration – Individual nerve block – Plexus block – Epidural administration Absorption / Elimination • LA drugs are absorbed into the systemic circulation from the site of administration – Rate of absorption depends on the site – Addition of adrenaline can delay absorption – After absorption, they are distributed rapidly and taken up by organs • Metabolized in the liver and excreted by the kidney • LA cross the placenta, but their effects are of minimal significance Systemic toxicity • If significant amounts of LA drugs are absorbed they can cause toxicity : – Nervous system: • Numbness and tingling over the circumoral area • Anxiety, Light-headedness, tinnitus • Loss of consciousness and convulsions – Heart • Direct myocardial depression and hypotension • Vasodilatation • Cardiac arrest Systemic toxicity can occur due to: • Inadvertent intravenous administration of LA drugs • Overdose if the following limits are exceeded: – Lignocaine plain – 4 mg / kg – Lignocaine with adrenaline – 7 mg / kg – Bupivacaine – 2 mg / kg Other Drugs (Miscellaneous category) • Steroids – Dexamethasone , Prednisone Dexamethasone • Is a potent synthetic member of the glucocorticoid class of steroid drugs • It acts as anti-inflammatory • Immunosuppresant • Can be taken orally and is more potent than the naturally occurring hormone ‘cortisol’ • Used to reduce pain and inflammation in – Rheumatoid arthritis Non-pharmacological treatments • Both physical and psychological factors affect our perception of pain. Treatments include: • Physical – Rest, Ice (cold), Compression, Elevation of injuries – Surgery (e.g. draining an abscess / fixing fractures) – Acupuncture, massage, physiotherapy – Ultrasound therapy – Transcutaneous electrical nerve stimulation (TENS) Psychological treatments • Adequate explanation • Reassurance that their pain will be addressed • Counseling – Individual – Family • This is particularly important when dealing with patients with cancer pain • Cognitive Behavioural Therapy (CBT) – for chronic non-cancer pain Conclusion – Adequate control of pain requires: • An understanding the mechanisms of pain: – Nociceptive or neuropathic pain • An understanding of the drugs that help to control it: – Mechanism of action – Pharmacokinetics (Absorption and time to onset and peak action and elimination) – Side effect profile This talk was originally prepared by: Ramani Vijayan, M.D. Kuala Lumpur, Malaysia International Pain School Talks in the International Pain School include the following: Physiology and pathophysiology of pain Nilesh Patel, PhD, Kenya Assessment of pain & taking a pain history Yohannes Woubished, M.D, Addis Ababa, Ethiopia Clinical pharmacology of analgesics and non-pharmacological treatments Ramani Vijayan, M.D. Kuala Lumpur, Malaysia Postoperative – low technology treatment methods Dominique Fletcher, M.D, Garches & Xavier Lassalle, RN, MSF, Paris, France Postoperative– high treatment technology methods Narinder Rawal, M.D. PhD, FRCA(Hon), Orebro, Sweden Cancer pain– low technology treatment methods Barbara Kleinmann, MD, Freiburg, Germany Cancer pain– high technology treatment methods Jamie Laubisch MD, Justin Baker MD, Doralina Anghelescu MD, Memphis, USA Palliative Care Jamie Laubisch MD, Justin Baker MD, Memphis, USA Neuropathic pain - low technology treatment methods Maija Haanpää, MD, Helsinki & Aki Hietaharju, Tampere, Finland Neuropathic pain – high technology treatment methods Maija Haanpää, M.D., Helsinki & Aki Hietaharju, M.D., Tampere, Finland Psychological aspects of managing pain Etleva Gjoni, Germany Special Management Challenges Debra Gordon, RN, DNP, FAAN, Seattle, USA International Pain School The project is supported by these organizations: