* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PLASMA FRACTIONS (cont`d)
Psychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Drugs Used in Disorders
of Coagulation
MECHANISMS OF BLOOD
COAGULATION
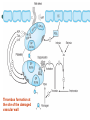
Hemostasis: spontaneous arrest of bleeding
from damaged blood vessel.
Immediate hemostatic response of damaged
vessel is vasospasm.
Within seconds, platelets stick to exposed
collagen of damaged endothelium (platelet
adhesion) & to each other (platelet
aggregation).
Fibrin reinforcement results from local stimuli
to blood coagulation: exposed collagen of
damaged vessels & membranes & released
contents of platelets.
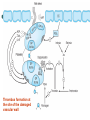
Thrombus formation at
the site of the damaged
vascular wall
White Thrombus Versus Red
Thrombus
White thrombus forms initially in high-pressure
arteries by adherence of circulating platelets to
areas of abnormal endothelium →↓ arterial flow
→ fibrin formation → red thrombus.
Red thrombus can form around white thrombus
in low-pressure veins, initially by adherence of
platelets → formation of long tail consisting of
fibrin network in which red cells are enmeshed.
These tails become detached easily & travel as
emboli to pulmonary arteries PE
Arterial Thrombus Versus
Venous Thrombus
Although all thrombi are mixed,
platelet nidus dominates arterial
thrombus & fibrin tail venous
thrombus.
Arterial thrombi cause serious disease
by producing local occlusive
ischemia amputation;
venous thrombi, by giving rise to
distant embolization PE sudden
death
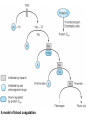
Blood Clotting Factors & Drugs That Affect Them
A model of blood coagulation.
Role of thrombin
Has central role in hemostasis & many functions:
1.
2.
3.
4.
5.
In clotting, thrombin proteolytically: fibrinogen → fibrin
activates many upstream clotting factors, leading to more thrombin
generation,
Activates factor XIII, a transaminase that cross-links the fibrin polymer
& stabilizes the clot.
Thrombin is a potent platelet activator & mitogen.
Thrombin also exerts anticoagulant effects by activating the protein C
pathway, which attenuates the clotting response
→ Under normal circumstances, repair of vascular injury occurs without
thrombosis & downstream ischemia;. Eventually vascular remodeling and
repair occur with reversion to the resting anticoagulant endothelial cell
phenotype
Antithrombin (AT) III
Is an endogenous anticoagulant
Inactivates the serine proteases IIa, IXa, Xa, XIa,
& XIIa.
The endogenous anticoagulants protein C &
protein S attenuate the blood clotting cascade by
proteolysis of the two cofactors Va & VIIIa.
Defects in natural anticoagulants result in an
increased risk of venous thrombosis.
The most common defect in the natural
anticoagulant system is a mutation in factor V
(factor V Leiden), which results in resistance to
inactivation by the protein C, protein S
mechanism.
clinically
useful
activators
useful in some
bleeding disorders
Basic Pharmacology of
Antithrombotic Agents
_______________
1. Anticoagulants (Heparin &
Warfarin)
HEPARIN:
UFH Chemistry & Source
Heparin: heterogeneous mixture of sulfated
mucopolysaccharides.
Prevents further thrombus growth, allowing
the body’s own thrombolytic system to
dissolve clot.
Regular (UFH) is standardized as units of
activity by bioassay.
Commercial heparin is extracted from
porcine intestinal mucosa & bovine lung.
UFH is eliminated by lungs, RES
(macrophages of liver), & kidney.
Mechanism of UFH action
UFH activates plasma antithrombin III (AT
III).
Heparin molecules have unique
pentasaccharide sequence, allowing it to
bind & catalyze ATIII.
This complex inactivates factors, IXa, Xa, &
IIa (thrombin)
Special requirement for inactivation of
thrombin: heparin acts as bridge to which
both thrombin & ATIII bind.
UFH vs LMWH
High-molecular-weight fractions of heparin with high affinity
for antithrombin markedly inhibit blood coagulation by
inhibiting all 3 clotting factors involved, especially thrombin
and factor Xa.
the shorter-chain low-molecular-weight (LMWH) inhibit
activated factor X but have less effect on thrombin than
UFH.
Unfractionated heparin (UFH) has a molecular weight range
of 5000-30,000.
LMWHs such as enoxaparin, dalteparin, & tinzaparin are
effective in several thromboembolic conditions.
LMWHs in comparison with UFH have equal efficacy,
increased bioavailability from the SQ injection, & less
frequent dosing (1-2 times daily).
Laboratory Monitoring for
UFH
Close monitoring of the activated partial thromboplastin
time (aPTT) is necessary in patients receiving UFH.
Levels of UFH may also be determined by protamine titration
(therapeutic levels 0.2-0.4 unit/mL) or anti-Xa units
(therapeutic levels 0.3-0.7 unit/mL).
Weight-based dosing of the LMW heparins results in
predictable pharmacokinetics & plasma levels in patients with
normal renal function→ Thus,
levels are not generally measured except in the setting of
renal insufficiency, obesity, & pregnancy by anti-Xa units.
UFH Toxicity
1. The major adverse effect is bleeding
(elderly women & pts. w renal insufficiency
are at the highest risk)
2. Heparin is of animal origin & should be
used cautiously in patients with allergy.
3. Increased loss of hair (reversible alopecia)
4. Long-term heparin therapy: osteoporosis
5. Hyperkalemia (decreases aldosterone)
6. Heparin-induced thrombocytopenia
(HIT).
Heparin-induced
thrombocytopenia (HIT)
a systemic hypercoagulable state that occurs in 1-4% of
individuals treated with UFH for a minimum of 7 days.
Surgical patients are at greatest risk.
The risk is higher in individuals treated with UFH of bovine
origin
HIT is lower in those treated exclusively with LMWH.
HIT is related to thrombotic events: venous thrombosis,
occlusion of peripheral or central arteries
The following points should be
considered in all patients receiving
heparin :
platelet counts should be performed
frequently.
thrombocytopenia should be considered
to be HIT.
any new thrombus can be result of
heparin.
HIT treated by discontinuance of
heparin & administration of direct
thrombin inhibitor or fondaparinux
Administration & Dosage
(cont’d)
The synthetic pentasaccharide molecule
fondaparinux avidly binds antithrombin with high
specific activity, resulting in efficient inactivation of
factor Xa.
Fondaparinux long half-life of 15 hours,
once-daily dosing by SC
It is used once-daily SQ for prevention &
treatment of venous thromboembolism,
The use of fondaparinux as an alternative
anticoagulant in HIT is currently being tested in
clinical trials.
Reversal of Heparin Action
Protamine: highly basic peptide that combines
with heparin as ion pair to form a stable complex
devoid of anticoagulant activity.
For every 100 units of heparin remaining in
patient, 1 mg of protamine sulfate is used IV; the
rate of infusion should not exceed 50 mg in any
10-min. period.
Excess protamine must be avoided; it also has
an anticoagulant effect.
Blood or FFP (fresh frozen plasma) are
sometimes necessary
Protamine will not reverse the activity of
fondaparinux
ORAL ANTICOAGULANTS :
WARFARIN & THE COUMARIN
ANTICOAGULANTS
Introduction
“warfarin” from the Wisconsin Alumni Research
Foundation.
is also widely used as a rodenticide.
is generally used as sodium salt & has 100%
bioavailability.
> 99% is bound to plasma albumin → small Vd,
long t1/2 (36 hr) & lack of urinary excretion of
unchanged drug.
Levorotatory S-warfarin is 4 times > potent than
the R-warfarin.
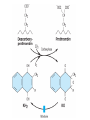
Structural formulas of several oral anticoagulant drugs and of vitamin K. The
carbon atom of warfarin shown at the asterisk is an asymmetric center
Warfarin
Mechanism of Action
Blocks γ-carboxylation of several glutamate residues in
prothrombin & factors VII, IX, & X, as well as the
proteins C and S.
The blockade results in incomplete molecules that are
biologically inactive in coagulation.
This carboxylation is physiologically coupled with the
oxidative deactivation of vitamin K.
Warfarin prevents reductive metabolism of inactive
vitamin K epoxide back to its active hydroquinone
form.
Warfarin
Mechanism of Action
There is an 8- to 12-hour delay in the action of
warfarin.
Its anticoagulant effect results from a balance
between partially inhibited synthesis & unaltered
degradation of the 4 vitamin K-dependent clotting
factors.
The resulting inhibition of coagulation is
dependent on their degradation half-lives in the
circulation: 6, 24, 40, & 60 hours for factors VII, IX,
X, & II, respectively.
Larger initial doses of warfarin (up to 0.75 mg/kg)
hasten the onset of the anticoagulant effect.
Warfarin Administration &
Dosage
Initiation is recommended at dose of 5-10 mg daily.
The therapeutic range for oral anticoagulant therapy is
defined in terms of an international normalized ratio
(INR).
The INR is the prothrombin time (PT) ratio (patient
prothrombin time/mean of normal prothrombin time for
lab)ISI,
The ISI serves to relate measured prothrombin times to
WHO reference standard thromboplastin
The recommended INR for prophylaxis & treatment of
thrombotic disease is 2-3.
Patients with some types of artificial heart valves (eg,
tilting disk) or other medical conditions increasing
thrombotic risk have a recommended range of 2.5-3.5
Warfarin Toxicity
1. Bleeding – the most dangerous.
2. Warfarin crosses the placenta readily & can
cause hemorrhagic disorder in the fetus.
3. birth defect: abnormal bone formation (fetal
proteins with γ-carboxyglutamate residues found in bone and blood
may be affected).Thus,
warfarin should never be
administered during pregnancy.
4. Cutaneous necrosis, infarction of breast,
fatty tissues, intestine, & extremities due to
venous thrombosis (↓activity of protein C).
Warfarin Drug Interactions
1. Pharmacokinetic mechanisms
enzyme induction,
enzyme inhibition,
↓plasma protein binding.
2. Pharmacodynamic mechanisms
synergism (impaired hemostasis, ↓ clotting
factor synthesis as in hepatic disease),
competitive antagonism (vitamin K).
and an altered physiologic control loop for
vitamin K (hereditary resistance to oral
anticoagulants)
Warfarin Drug Interactions
The most serious interactions are those that
↑ anticoagulant effect & risk of bleeding.
The most dangerous are pharmacokinetic
interactions with phenylbutazone and
sulfinpyrazone: not only augment
hypoprothrombinemia but also ↓platelet
function & may induce peptic ulcer disease.
The use of drug that interacts with warfarin
is not absolute contraindication to addition
of warfarin.
Pharmacokinetic & pharmacodynamic drug & body interactions
with oral anticoagulants.
Increased Prothrombin Time
Pharmacokinetic
Pharmacodynamic
Decreased Prothrombin Time
Pharmacokinetic
Pharmacodynamic
Amiodarone
Drugs
Barbiturates
Drugs
Cimetidine
Aspirin (high doses)
Cholestyramine
Diuretics
Disulfiram
Cephalosporins,
third-generation
Rifampin
Vitamin K
Metronidazole1
Heparin
Body factors
Fluconazole1
Body factors
Hereditary
resistance
Phenylbutazone1
Hepatic disease
Hypothyroidism
Sulfinpyrazone1
Hyperthyroidism
Trimethoprimsulfamethoxazole1
1Stereoselectively
inhibits oxidative metabolism of (S)-warfarin enantiomorph of racemic warfarin.
Warfarin -Disease
Interactions
augment warfarin pharmacodynamically by :
1. ↑ turnover rate of clotting factors:
- hepatic disease,
- fever,
hyperthyroidism
2. by reducing vitamin K absorption - diarrhea
reduce warfarin effect pharmacodynamically:
- hereditary resistance (mutation of vitamin K
reactivation cycle molecules)
- hypothyroidism (↓turnover rate of clotting
factors).
Reversal of warfarin action:
stopping the drug
oral or parenteral vitamin K1
(phytonadione),
fresh-frozen plasma,
prothrombin complex concentrates
such as Bebulin & Proplex T,
recombinant factor VIIa (rFVIIa).
FIBRINOLYTIC DRUGS
Pharmacology
Fibrinolytic drugs rapidly lyse thrombi by
catalyzing formation of serine protease
plasmin from its precursor, plasminogen.
Streptokinase: protein (but not enzyme in
itself) synthesized by streptococci that
combines with the proactivator plasminogen
to catalyze conversion of inactive
plasminogen to active plasmin.
Pharmacology
Urokinase: human enzyme synthesized by kidney that
directly converts plasminogen to active plasmin.
Plasmin formed inside thrombus by these activators is
protected from plasma antiplasmins, which allows it to lyse
thrombus from within.
Anistreplase (anisoylated plasminogen streptokinase
activator complex; APSAC) consists of a complex of purified
human plasminogen & bacterial streptokinase that has been
acylated to protect the enzyme's active site.
When administered, the acyl group spontaneously
hydrolyzes, freeing the activated streptokinase-proactivator
complex.
This product has been recently discontinued in the USA
Pharmacology (cont’d)
Plasminogen can also be activated endogenously by tissue
plasminogen activators (t-PA): preferentially activate
plasminogen that is bound to fibrin, which confines
fibrinolysis to formed thrombus.
Human t-PA is manufactured as alteplase by means of
recombinant DNA technology.
Reteplase is another recombinant human t-PA from which
several amino acid sequences have been deleted. It is less
expensive than t-PA. Because it lacks the major fibrinbinding domain, reteplase is less fibrin-specific than t-PA.
Tenecteplase is a mutant form of t-PA that has a longer
half-life, & it can be given as an IV bolus. Tenecteplase is
slightly more fibrin-specific than t-PA.
Fibrinolytics in AMI:
thrombolytic therapy is still very important where
PCI is not readily available.
The proper selection of patients for thrombolytic
therapy is critical:
Patients with ST-segment elevation & bundle
branch block on ECG have the best outcomes.
The greatest benefit for thrombolytic therapy: when
given early, within 6 hours after onset
Thrombolytic drugs ↓ mortality of AMI.
Adjunctive drugs such as aspirin, heparin, ß
blockers, & ACEIs ↓ mortality even further.
Indications & Dosage of
Fibrinolytics
-
-
-
Administration of fibrinolytic drugs by IV route is
indicated in cases of
pulmonary embolism (PE) with hemodynamic
instability,
severe deep venous thrombosis (DVT) such as
the superior vena caval syndrome,
ascending thrombophlebitis of the iliofemoral
vein with severe lower extremity edema.
intra-arterially for peripheral vascular disease.
Indications & Dosage of
Fibrinolytics (cont’d)
In AMI: Streptokinase - IV infusion of a loading dose of 250,000
units → 100,000 units/h for 24-72 hours.
Patients with antistreptococcal antibodies can develop fever,
allergic reactions, & therapeutic resistance.
Alteplase (t-PA) is given by IV infusion of 60 mg over the first
hour → 40 mg at a rate of 20 mg/h.
A single course of fibrinolytic drugs is expensive: hundreds of
dollars for streptokinase & thousands for urokinase & t-PA.
Recombinant t-PA has also been approved for use in acute
ischmic (non-hemorrhagic) stroke within 3 hours of symptom
onset. The recommended dose is 0.9 mg/kg, not to exceed 90
mg, with 10% given as a bolus & the remainder during a 1 hour
infusion.
ANTIPLATELET AGENTS
1.
Platelet function is regulated by three
categories of substances.
Agents generated outside the platelet that
interact with platelet membrane receptors,
eg, catecholamines, collagen, thrombin, and prostacyclin.
2.
Agents generated within the platelet that
interact with membrane receptors,
eg, ADP, prostaglandin D2, prostaglandin E2, and serotonin.
3.
Agents generated within the platelet that act
within the platelet, eg, prostaglandin endoperoxides and
thromboxane A2, the cyclic nucleotides cAMP and cGMP, and
calcium ion.
Thrombus formation at
the site of the damaged
vascular wall
Targets for platelet inhibitory
drugs:
inhibition of prostaglandin synthesis
(aspirin),
inhibition of ADP-induced platelet
aggregation (clopidogrel, ticlopidine),
blockade of GP IIb/IIIa receptors on
platelets (abciximab, tirofiban, &
eptifibatide).
dipyridamole & cilostazol are additional
antiplatelet drugs
ASPIRIN
The prostaglandin thromboxane A2 (TxA2)
causes platelets to aggregate
Aspirin inhibits synthesis of TxA2 by
irreversible acetylation of COX enzyme.
Other salicylates & NSAIDs also inhibit COX
but have a shorter duration of inhibitory
action because they cannot acetylate COX;
that is, their action is reversible.
Aspirin (cont’d)
The FDA has approved the use of 325 mg/d for
primary prophylaxis of MI but urges caution in this
use of aspirin by the general population except
when prescribed as an adjunct to risk factor
management by smoking cessation & lowering of
blood cholesterol & BP.
Meta-analysis of many published trials of aspirin &
other antiplatelet agents confirms the value of this
intervention in the secondary prevention of
vascular events among patients with a history of
vascular events.
CLOPIDOGREL &
TICLOPIDINE (Thienopyridine derivatives)
-
Irreversibly inhibit ADP binding to its
receptor on platelets.
used to prevent vascular events in
patients with:
transient ischemic attacks (TIA) & strokes,
unstable angina pectoris.
now considered standard practice in
patients undergoing placement of a
coronary stent.
CLOPIDOGREL &
TICLOPIDINE
Adverse Effects
Ticlopidine
nausea, dyspepsia, diarrhea (20%),
hemorrhage (5%)
Most seriously: Leukopenia (1%) regular monitoring
of WBCs during the first 3 months is necessary
thrombotic thrombocytopenic purpura (TTP)
Clopidogrel – fewer SE than with ticlopidine
Rarely: neutropenia.
TTP
thrombotic thrombocytopenic purpura
A rapidly fatal or occasionally
protracted disease with varied
symptoms in addition to purpura,
including signs of central nervous
system involvement, due to formation
of fibrin or platelet thrombi in arterioles
and capillaries in many organs.
Synonym: Moschcowitz' disease.
CLOPIDOGREL &
TICLOPIDINE
Dosage
Because of its superior side effect profile and
dosing requirements, clopidogrel is preferred over
ticlopidine.
The antithrombotic effects of clopidogrel are dosedependent; within 5 hours after an oral loading
dose of 300 mg, 80% of platelet activity will be
inhibited.
The maintenance dose of clopidogrel is 75 mg/d,
which achieves maximum platelet inhibition.
The duration of the antiplatelet effect is 7-10 days
BLOCKADE OF PLATELET
GP IIB/IIIA RECEPTORS
A new class of antiplatelet agents for use in
patients with acute coronary syndromes
(ACCs).
The IIb/IIIa complex functions as a receptor
mainly for fibrinogen and vitronectin but also
for fibronectin & von Willebrand factor.
Activation of this receptor complex is the
"final common pathway" for platelet
aggregation.
BLOCKADE OF PLATELET
GP IIB/IIIA RECEPTORS
Abciximab, a chimeric monoclonal antibody directed
against the IIb/IIIa complex including the vibronectin
receptor, was the first agent approved in this class of
drugs- approved for use in PCI & ACCs.
Eptifibatide is an analog of the sequence at the
extreme carboxyl terminal of the delta chain of
fibrinogen, which mediates the binding of fibrinogen to
the receptor.
Tirofiban is a smaller molecule with similar properties.
Eptifibatide & tirofiban inhibit ligand binding to the
IIb/IIIa receptor by their occupancy of the receptor but
do not block the vibronectin receptor.
All three agents are administered parenterally
ADDITIONAL ANTIPLATELETDIRECTED DRUGS
Dipyridamole is a vasodilator that inhibits platelet function by
inhibiting adenosine uptake & cyclic GMP phosphodiesterase
activity.
Primarily used in combination with aspirin to prevent
cerebrovascular ischemia.
may also be used in combination with warfarin for primary
prophylaxis of thromboemboli in patients with prosthetic heart
valves.
A combination of dipyridamole complexed with 25 mg of aspirin is
now available for secondary prophylaxis of cerebrovascular
disease.
Cilostazol is a newer PDE inhibitor that promotes vasodilation &
inhibition of platelet aggregation. Cilostazol is used primarily to
treat intermittent claudication.
Figure 2. Mechanism of action of
dipyridamole on platelet activation
CLINICAL
PHARMACOLOGY OF
DRUGS USED TO
PREVENT CLOTTING
VENOUS THROMBOSIS
Risk Factors
A. INHERITED DISORDERS
Tendency to form thrombi (thrombophilia)
Deficiencies (loss of function mutations) in the
natural anticoagulants antithrombin, protein C,
& protein S account for thrombosis.
Gain of function mutations such as the factor
V Leiden mutation & the prothrombin 20210
mutation,
Elevated clotting factor & cofactor levels, &
hyperhomocysteinemia
VENOUS THROMBOSIS
(cont’d)
B. ACQUIRED DISEASE
↑ risk of thromboembolism:
atrial fibrillation,
placement of mechanical heart valves
prolonged bed rest,
high-risk surgical procedures,
presence of cancer
antiphospholipic antibody syndrome
drugs may function as synergistic risk
factors in concert with inherited risk factors
(e.g., women who have the factor V Leiden
mutation and take oral contraceptives)
Antithrombotic Management
PREVENTION
Primary prevention of venous thrombosis ↓ incidence
of & mortality rate from pulmonary emboli.
Heparin & warfarin may be used to prevent venous
thrombosis.
SQ low-dose UFH, LMWH, or fondaparinux provides
effective prophylaxis.
Warfarin is also effective but requires laboratory
monitoring of the prothrombin time.
Antithrombotic Management
B. TREATMENT OF ESTABLISHED venous
thrombosis
initiated with UFH or LMWH for the first 5-7 days, with
an overlap with warfarin.
Once therapeutic effects of warfarin have been
established, therapy with warfarin is continued for a
minimum of 3-6 months.
Warfarin readily crosses the placenta. It can cause
hemorrhage at any time during pregnancy as well as developmental
defects when administered during the first trimester
→ venous thromboembolic disease in pregnant women
is generally treated with heparin SQ
ARTERIAL THROMBOSIS
Activation of platelets is considered an
essential process for arterial thrombosis
Thus, treatment with platelet-inhibiting drugs
such as aspirin, ticlopidine & clopidogrel is
indicated in patients with transient ischemic
attacks & strokes or unstable angina ´
myocardial infarction.
In angina & infarction, these drugs are often
used in conjunction with ß-blockers, calcium
channel blockers, & fibrinolytic drugs.
DRUGS USED IN BLEEDING
DISORDERS
VITAMIN K: Mechanism of
action
confers biologic activity upon prothrombin &
factors VII, IX, & X by participating in their
postribosomal modification.
fat-soluble substance found primarily in leafy
green vegetables.
dietary requirement is low, because it is
additionally synthesized by intestinal bacteria.
Vitamin K1 (phytonadione) is found in food.
Vitamin K2 (menaquinone) is synthesized by
intestinal bacteria.
VITAMIN K: Administration & Clinical
Uses
Vitamins K1 & K2 require bile salts for GIT
absorption.
Vitamin K1 is available in 5 mg tablets & 50 mg
ampules.
The effect is delayed for 6 hours. Complete in 24
hrs.
IV administration of vitamin K1 should be slow (rapid
infusion can produce dyspnea, chest & back pain &
death).
in all newborns to prevent the hemorrhagic disease
of vitamin K deficiency esp. if premature.
in reversal of warfarin effect.
Vitamin K deficiency frequently occurs in
hospitalized patients in intensive care units
because of:
poor diet, parenteral nutrition, recent
surgery, multiple antibiotic therapy, and
uremia.
Severe hepatic failure results in diminished
protein synthesis and a hemorrhagic
diathesis that is unresponsive to vitamin K.
PLASMA FRACTIONS
Spontaneous bleeding occurs when factor
activity is <than 5-10% of normal.
Factor VIII deficiency (classic hemophilia, or
hemophilia A) & factor IX deficiency
(Christmas disease, or hemophilia B)
account for most of the heritable
coagulation defects.
Concentrated plasma fractions are available
for the treatment of these deficiencies.
PLASMA FRACTIONS
(cont’d)
Transmission of viral diseases such as hepatitis B,
C &HIV is ↓ or eliminated by pasteurization & by
extraction of plasma with solvents & detergents.
However, this treatment does not remove other
potential causes of transmissable diseases such as
prions → recombinant clotting factor preparations
Intermediate purity factor VIII concentrates (as
opposed to recombinant or high purity
concentrates) contain significant amounts of von
Willebrand factor.
Humate-P is a factor VIII concentrate that is
approved by the FDA for the treatment of bleeding
associated with von Willebrand disease.
PLASMA FRACTIONS
(cont’d)
Desmopressin acetate (arginine
vasopressin) ↑ factor VIII activity of
patients with mild hemophilia A or von
Willebrand's disease.
Intranasal formulations are available
PLASMA FRACTIONS
(cont’d)
Freeze-dried concentrates of plasma containing
prothrombin, factors IX & X, & varied amounts of factor VII
(Proplex, etc) are commercially available for treating
deficiencies of these factors.
Some preparations of factor IX concentrate contain
activated clotting factors, which has led to their use in
treating patients with inhibitors or antibodies to factor VIII
or factor IX (to arrest hemorrhage)
Recombinant activated factor VII (NovoSeven) is used to
treat coagulopathy associated with liver disease & major
blood loss in trauma & surgery.
These recombinant & plasma-derived factor concentrates
are very expensive
PLASMA FRACTIONS
(cont’d)
Cryoprecipitate is a plasma protein fraction obtainable from
whole blood.
It is used to treat deficiencies or qualitative abnormalities of
fibrinogen, such as that which occurs with DIC & liver
disease.
A single unit of cryoprecipitate contains 300 mg of fibrinogen.
Cryoprecipitate may also be used for patients with factor VIII
deficiency & von Willebrand disease
The concentration of factor VIII & von Willebrand factor in
cryoprecipitate is less than in concentrated plasma fractions.
Cryoprecipitate is not treated to ↓ risk of viral exposure.
Rh-negative women with potential for childbearing should
receive only Rh-negative cryoprecipitate because of possible
contamination of the product with Rh-positive blood cells.
Therapeutic products for the treatment of coagulation disorders
Factor
Deficiency State
I
Hypofibrinogenemia
II
Prothrombin
deficiency
V
Factor V def.
VII
VIII
Factor VII deficiency
Hemophilia A
Hemostatic
Levels
t1/2
Replacement Source
4 days
Cryoprecipitate
FFP
30-40%
3 days
Prothrombin complex
concentrates (intermediate
purity factor IX concentrates)
20%
1 day
FFP
4-6
hours
FFP
Prothrombin complex
concentrates (intermediate
purity factor IX concentrates)
Recombinant fr VIIa
12
hours
Recombinant factor VIII
products
Plasma-derived high purity
concentrates
Cryoprecipitate1
Some patients with mild
deficiency will respond to
DDAVP
1 g/dL
30%
30-50%
100% for
major
bleeding or
trauma
30-50%
100% for
major
24 hours
bleeding or
trauma
Recombinant factor IX
products
Plasma-derived high
purity concentrates
IX
Hemophilia B
Christmas
disease
X
Stuart-Prower
defect
25%
36 hours
FFP
Prothrombin complex
concentrates
XI
Hemophilia C
30-50%
3 days
FFP
XII
Hageman defect
Not
required
Von
Von Willebrand
Willebra
30%
disease
nd
XIII
Factor XIII
deficiency
5%
Treatment not necessary
Intermediate purity factor
VIII concentrates that
Approxim contain von Willebrand
ately 10
factor
hours
Some patients respond to
DDAVP
Cryoprecipitate1
6 days
FFP
Cryoprecipitate
FIBRINOLYTIC INHIBITORS:
AMINOCAPROIC ACID
Aminocaproic acid (EACA), chemically similar
to amino acid lysine, is synthetic inhibitor of
fibrinolysis:
competitively inhibits plasminogen activation.
Used orally or by IV infusion.
Clinical uses: as adjunctive therapy in
hemophilia, & for bleeding from other causes.
Tranexamic acid is an oral analog of
aminocaproic acid and has the same
properties
SERINE PROTEASE
INHIBITORS: APROTININ
Aprotinin: serine protease inhibitor ("serpin")
- inhibits fibrinolysis by free plasmin and also
plasmin-streptokinase complex.
is currently approved for use in patients
undergoing coronary artery bypass grafting
(CABG) who are at high risk of excessive
blood loss.
ADRs: ↑ risk of MI, stroke, & renal damage,
possible association with anaphylaxis →
a small test dose is recommended before the
full therapeutic dose is given