* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Basis of Life – Biochemistry - Har
Radical (chemistry) wikipedia , lookup
DNA-encoded chemical library wikipedia , lookup
Chemical biology wikipedia , lookup
Biomolecular engineering wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Carbohydrate wikipedia , lookup
Abiogenesis wikipedia , lookup
List of types of proteins wikipedia , lookup
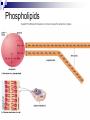
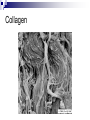
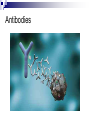
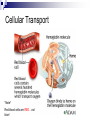
Chemical Basis of Life – Biochemistry What is Biochemistry Biochemistry is the study of the chemical interactions of living things. Living things require millions of chemical reactions within the body, just to survive. Biochemists study the structures and physical properties of biological molecules. Often are involved in the manufacture of new drugs and medical treatments Chemical Organization Matter – anything that has mass and takes up space Atoms – the smallest, stable unit of matter Made of protons (+ charge), neutrons (neutral charge), and electrons (- charge) Protons and neutrons are located in the nucleus of the atom Electrons are in an electron cloud outside of the nucleus Atomic Structure Elements – all matter composed of them; found on periodic table Atomic number Oxygen, carbon, hydrogen, and nitrogen make up more than 95% of the human body Atomic number – unique to each element; equals number of protons and electrons in a neutral atom Isotopes – atoms of same atomic number, but different atomic mass due to differing amounts of neutrons; normal in nature; average of isotopes = atomic mass on periodic table Electron Shell/ Electron Cloud – electrons are found moving around the nucleus in the “cloud” Atomic mass Chemical Bonding Molecule – particle formed when two or more atoms chemically combine (H2) Compound – particle formed when two or more atoms of different elements chemically combine (H2O) Molecular formulas – depict the elements present and the number of each atom present in the molecule or compound H2 C6H12O6 H2O Ionic Bonds Ionic Bond – attraction between a cation and an anion; formed when electrons are transferred from one atom to another atom Cation – atoms that have lost electrons and have a net positive charge Anion – atoms that have gained electrons and have a net negative charge Covalent Bonds - Formed when atoms share electrons Hydrogen atoms form single bonds Oxygen atoms form two bonds Nitrogen atoms form three bonds Carbon atoms form four bonds H―H O=O N≡N O=C=O Nonpolar covalent – atoms remain electrically neutral and share electons equally Polar covalent – an unequal sharing of electrons between atoms causes a slightly positive end and a slightly negative end Chemical Reactions Metabolism = all the chemical reactions occurring in the body. 2H2 + O2 2H2O reactants products Chemical reactions occur when chemical bonds form or break among atoms, ions, or molecules Hydrogen Bonds a weak attraction between the positive end of one polar molecule and the negative end of another polar molecule formed between water molecules important for protein and nucleic acid structure Types of Reactions Synthesis Reaction – more complex chemical structure is formed; Anabolism – the synthesis of new compounds in the body A + B AB Decomposition Reaction – chemical bonds are broken to form a simpler chemical structure; Catabolism – decomposition of complex molecules within cells AB A + B Double Replacement/Exchange/Metathesis Reaction – chemical bonds are broken and new bonds are formed AB + CD AD + CB Reversible Reaction – the products can change back to the reactants; at equilibrium the rates of the two reactions are in balance A+B AB Enzymes or catalysts Most chemical reactions do not occur spontaneously; they need something to speed it up or make it happen The amount of energy required to start reactions is the activation energy. Enzymes belong to a class of substances called catalysts – compounds that accelerate chemical reactions without themselves being permanently changed. If it releases energy it is exergonic. If more energy is required to begin the reaction than is released, it will absorb energy as a whole and is endergonic. Organic vs. Inorganic Organic molecules contain C and H usually larger than inorganic molecules dissolve in water and organic liquids carbohydrates, proteins, lipids, and nucleic acids Inorganic molecules generally do not contain C usually smaller than organic molecules usually dissociate in water, forming ions water, oxygen, carbon dioxide, and inorganic salts Inorganic Substances Water most abundant compound in living material two-thirds of the weight of an adult human major component of all body fluids medium for most metabolic reactions important role in transporting chemicals in the body absorbs and transports heat Oxygen (O2) used by organelles to release energy from nutrients in order to drive cell’s metabolic activities necessary for survival Inorganic Substances Carbon dioxide (CO2) waste product released during metabolic reactions must be removed from the body Inorganic salts abundant in body fluids sources of necessary ions (Na+, Cl-, K+, Ca2+, Mg2+ etc.) play important roles in metabolism Acids, Bases, & Salts Acids – any substance that dissociates in solution to release hydrogen ions (H+); the pH is the H+ concentration HCl H+ + ClBases – A substance that removes the hydrogen ions from a solution; most dissociate to liberate a hydroxide ion (OH-) NaOH Na+ + OHBuffers – compounds that stabalize pH by either removing or replacing hydrogen ions Antacids like Tums, Rolaids; baking soda (sodium bicarbonate) Salts – electrolytes formed between the reaction of an acid and a base; composed of oppositely charged ions Electrolytes can conduct an electrical current in solution Important electrolytes for body functions: Na+, K+, Ca2+, Cl-, Mg2+ Macromolecules of Cells Macro = large 4 types of macromolecules in cellular biology 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Macromolecule #1: Carbohydrates Sugars and groups of sugars Purposes: energy and structure Includes three types: Monosaccharide (1 sugar – quick energy) Disaccharide (2 sugars – short storage) Polysaccharide (many sugars – energy long storage & form structures) Macromolecule #1: Carbohydrates Polysaccharide Examples: Glycogen—glucose polymer stored for future energy needs. Found in liver, muscle and sperm, etc. Cellulose—glucose polymer used to form fibers for plant structures. Humans can’t digest (fiber). Most abundant organic molecule. Chitin—glucose polymer for exoskeletons of some crustaceans & insects. Polysaccharides Polysaccharides Macromolecule #2: Lipids Insoluble in water (think oil & water) Types: 1-Fats: triglycerides (fats & oils) (long-term energy storage, insulation) Building blocks are fatty acids and glycerol 2-phospholipids (primary component of cell membrane) A glycerol and two fatty acids linked to a nonlipid group by a phosphate group 3-steroids (cell signaling) cholesterol molecules modified to form sex hormones. (e.g. testosterone, estrogen, etc.) Triglycerides Phospholipids Steroids Macromolecule #3: Proteins Probably the most complicated of all biological molecules. Serve the most varied purposes, including: Support structural proteins (e.g., keratin, collagen) Enzymes speed up chemical reactions Transport cell membranes channels, transporters in blood (e.g., Hemoglobin) Defense antibodies of the immune system Hormones cell signaling (e.g., insulin) Motion contractile proteins (e.g., actin, myosin) Collagen Antibodies Cellular Transport *Note* Red blood cells are RED…not blue! Motion Actin & myosin fibers in muscles Macromolecule #3: Proteins The building blocks of proteins are AMINO ACIDS. There are only 20 types of Amino Acids. There are millions of different proteins, and they are all built from different combinations of the 20 amino acids. Amino acids join together to form peptides, polypeptides, and polypeptide chains. Macromolecule #4: Nucleic Acids Nucleotides: building blocks of nucleic acids. Each nucleotide contains (a) phosphate molecule, (b) nitrogenous base, and (c) 5-carbon sugar Several types of nucleic acids, including: DNA: deoxyribonucleic acid Genetic material, double stranded helix RNA: ribonucleic acid Genetic material, single stranded ATP: adenosine triphosphate High energy compound DNA Nucleotide structure THE BIG PICTURE Chemistry is essential for life…