* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Pharmacokinetics
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Plateau principle wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
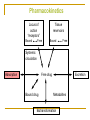
Basic Pharmacokinetics The time course of drug action Collected and Prepared By S.Bohlooli, PhD What is the goal of therapeutics? To achieve desired beneficail effect with Minimal side effects Governs dose-concentration part Rational apporach combines the principles of pharmacokinetics and pharmacodynamics to clarify the dose-effect relationship Governs concentration-effect part Pharmacokinetics “How the body handles a drug over time.” The Time-Dependent Roles of Drug Absorption, Distribution, and Elimination (Metabolism/Excretion) in Determining Drug Levels in Tissues. Pharmacokinetics Locus of action “receptors” Bound Tissue reservoirs Free Bound Free Systemic circulation Absorption Free drug Bound drug Excretion Metabolites Biotransformation Distribution Once a drug is absorbed into the bloodstream, it may be distributed into interstitial and cellular fluids. The actual pattern of drug distribution reflects various physiological factors and physicochemical properties of the drug. Factors affecting Distribution Size of the organ Blood flow to the tissue Solubility of the drug in the tissue Binding of the drug to macromolecules Phases of Distribution First phase reflects cardiac output and regional blood flow. Thus, heart, liver, kidney & brain receive most of the drug during the first few minutes after absorption. Next phase delivery to muscle, most viscera, skin and adipose is slower, and involves a far larger fraction of the body mass. Drug Reservoirs Body compartments where a drug can accumulate are reservoirs. They have dynamic effects on drug availability. plasma proteins as reservoirs (bind drug) cellular reservoirs Adipose (lipophilic drugs) Bone (crystal lattice) Transcellular (ion trapping) Protein Binding Passive movement of drugs across biological membranes is influenced by protein binding. Binding may occur with plasma proteins or with non-specific tissue proteins in addition to the drug’s receptors. ***Only drug that is not bound to proteins (i.e., free or unbound drug) can diffuse across membranes. Plasma Proteins albumin - binds many acidic drugs a1-acid glycoprotein for basic drugs The fraction of total drug in plasma that is bound is determined by its concentration, its binding affinity, and the number of binding sites. Bone Reservoir Tetracycline antibiotics (and other divalent metal ion-chelating agents) and heavy metals may accumulate in bone. They are adsorbed onto the bone-crystal surface and eventually become incorporated into the crystal lattice. Bone then can become a reservoir for slow release of toxic agents (e.g., lead, radium) into the blood. Adipose Reservoir Many lipid-soluble drugs are stored in fat. In obesity, fat content may be as high as 50%, and in starvation it may still be only as low as 10% of body weight. 70% of a thiopental dose may be found in fat 3 hr after administration. Thiopental • A highly lipid-soluble i.v. anesthetic. Blood flow to the brain is high, so maximal brain concentrations brain are achieved in minutes and quickly decline. Plasma levels drop as diffusion into other tissues (muscle) occurs. • Onset and termination of anesthesia is rapid. The third phase represents accumulation in fat (70% after 3 h). Can store large amounts and maintain anesthesia. Thiopental concentration (as percent of initial dose) 100 blood brain 50 muscle 1 10 100 minutes adipose 0 1000 GI Tract as Reservoir Weak bases are passively concentrated in the stomach from the blood because of the large pH differential. Some drugs are excreted in the bile in active form or as a conjugate that can be hydrolyzed in the intestine and reabsorbed. In these cases, and when orally administered drugs are slowly absorbed, the GI tract serves as a reservoir. Redistribution Termination of drug action is normally by biotransformation/excretion, but may also occur as a result of redistribution between various compartments. Particularly true for lipid-soluble drugs that affect brain and heart. Placental Transfer Drugs cross the placental barrier primarily by simple passive diffusion. Lipid-soluble, nonionized drugs readily enter the fetal bloodstream from maternal circulation. Rates of drug movement across the placenta tend to increase towards term as the tissue layers between maternal blood and fetal capillaries thin. Good Luck