* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Bonding
Biochemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Electronegativity wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Hydrogen bond wikipedia , lookup
Computational chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Atomic orbital wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Halogen bond wikipedia , lookup
History of molecular theory wikipedia , lookup
Electron configuration wikipedia , lookup
Metallic bonding wikipedia , lookup
Bond valence method wikipedia , lookup
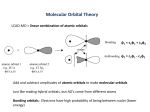
Molecular orbital wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Chemical bond wikipedia , lookup
Bent's rule wikipedia , lookup
Chem 1050 Chemical Bonding I and II Text: Petrucci, Harwood, Herring 8th Edition Chapter 11 Chemical Bonding I Basic Topics Suggested Text Problems Review problems: 4!8, 10 !18, 20, 21 Exercises: 27, 29, 33, 37, 39, 49, 51, 53, 55, 57, 59, 61, 63, 65, 67, 71, 73, 79, 81, 83, 94, 99 1. Draw Lewis structures for the following ionic compounds: NaF, Mg3P2, CaO 2. List the following bonds (A-B) in order of increasing ionic character. Assume that % ionic character is directly proportional to ∆EN (ENA-ENB ). Do not use the table in the text but predict the answers based on the general periodic trends and other data given in class. (a) C!F C!Br C!I C!Cl (b) (c) H!H C!N Na!F C!O C!F Si!F Cl!F S!F P!F 3. Define or explain coordinate covalent bond, resonance structures, resonance hybrid, free radical, incomplete octet and expanded octet. An example is a valuable addition to each explanation. 4. For the following molecules and ions: OF2, CCl4, PF6!, BF2Cl, HCN, ICl5, SnBr2, I3!, XeOF2, SeCl6, CO2, NF3, COS, SiCl3H, SOCl2, PO43!, NOF, BrF4+, ClNO2, BeH2, SO32!, ICl4! 1. 2. 3 4. 5. 6. 5. Draw a Lewis structure. (Include formal charges) Determine the electron group geometry and compound class. Sketch and name the shape. Indicate the approximate bond angles. Indicate whether or not each species has a permanent dipole moment (i.e. is polar or non!polar). Determine the hybridization of the central atom. (Chapter 12 topic, See Table 12.1 page 445) Draw three plausible structures for XeF2. Account for the fact that the linear structure is correct. 6. Draw plausible resonance structures for N2O (NNO), HNO3 (HONO2), CO32!. For each species, predict the bond order of each pair of bonding atoms. For N2O, predict the length of the NN bond and its bond energy given the following Bond bond length, pm bond energy/kJ@mol!1 N!N 145 163 N=N 123 418 N/N 109.8 946 7. Sketch the following molecules using VSEPR to determine the shape about each multiply bonded atom. Indicate the ideal bond angles in each structure. Complete the Lewis structures first. Atom frameworks are H3C!CO!CN, H2N!CH!NH. 8. Using bond dissociation energies in table 11.3 on page 422 in Petrucci and Harwood, 8th Edition, (A) estimate ∆H of the following reaction: C2H4(g) + 3 O2(g) ÿ 2 CO2(g) + 2 H2O(g) (B) estimate the bond dissociation energy of the nitrogen-to-oxygen bond in NO(g) if the enthalpy change for the reaction below is !756.3 kJ@mol!1. 2 NO(g) + 5 H2(g) ÿ 2 NH3(g) + 2 H2O(g) Calculate the % error given the measured value of 631 kJ@mol!1. Chapter 12 Chemical Bonding II Advanced Topics Review problems: 4, 5, 6, 9!14, 16, 17, 18 Exercises: 23, 27, 33, 35, 43, 45, 47, 49, 70 9. Draw Lewis structures for free radicals, CH3, ClO2 and NO2. 10. Use the simple valence bond method to describe bonding in H2Se and SnCl2. Step#1: Step#2: Step#3: Step#4: 11. Draw the Lewis structure Draw the valence shell orbital diagrams of the separated atoms Sketch only the orbitals of each atom that are involved in the bonding showing how they overlap to make the bonds. Indicate the predicted bond angles. Use valence bond theory and hybridization to describe bonding in BeH2, AlBr3, CH3Cl, NF3, SF4, XeF2, XeF4, (H2CO, HCN in which there are multiple bonds). Step#1: Draw the Lewis structure Step#2: Determine the number of electron groups of the central atom and the VSEPR shape. ( This determines the type of hybrid orbitals of the Step#3: Step#4: Step#5: central atom and their spacial geometry) Draw the valence shell orbital diagrams of the end atoms and the appropriate hybrid orbital diagram of the central atom. Sketch all the hybrid orbitals of the central atom but only the valence orbitals of the end atoms that are involved in the bonding showing how they overlap to make the bonds. Indicate the location of any lone pairs of the central atom. Indicate the type of bonds formed (Π or σ). Indicate the predicted bond angles. 12. Distinguish between the bonding and antibonding molecular orbitals formed by combining a pair of atomic orbitals. 13. Distinguish between Π (pi) and σ (sigma) bonding molecular orbitals. 14. Distinguish between normal π bonding and delocalized π bonding. 15. Draw valence molecular orbital diagrams (i.e. omitting inner shell orbitals) for the following homonuclear diatomic species, H2!, He2+, O2, N2!, C22!, Ne2+, (Na2, Mg2, P2, you can assume that third period diatomics form valence molecular orbitals similar to second period diatomics but with n=3) and the following heteronuclear diatomic species, CO, NO and BN (assume the same energy level diagram as Be2 to N2). Determine the bond order of each species. Indicate any species which will be unstable in the gas phase. Use Figure 12-20 on page 405 in Petrucci and Harwood, 7th Edition as a guide. 16. Draw two resonance structures for benzene C6H6. Use VSEPR to determine the shape of the molecule about each carbon. Determine the hybridization of each carbon atom and draw an valence orbital diagram to represent the carbon atoms. Sketch how the orbitals of carbon and hydrogen overlap to form sigma bonds. Sketch separately the delocalized Π bonding and draw an energy level diagram to show how the Π electrons occupy the available Π molecular orbitals. 17. Pick which of the following species possess delocalized molecular orbitals: CO2, NO3!, C2H4, O3, and C4H6 (H2C=CH-CH=CH2). 18. Explain what are non-bonding delocalized Π molecular orbitals. 19. Explain metallic bonding using the electron sea model and band theory. 20. Distinguish between conductors, semi-conductors and non-conductors using band theory. Answers: for most questions, partial answers given only. 2. Least ionic: C!I < C!Br < C!Cl < C!F :most ionic Least ionic: Cl!F < S!F < P!F < Si!F :most ionic Least ionic: H!H < C!N < C!O < C!F < Na!F :most ionic 4. formula electron group geometry Compound class name of shape bond angles polarity hybrid. OF2 tetrahedral AX2E2 V-shaped ~109.5 Polar sp3 CCl4 tetrahedral AX4 tetrahedral ~109.5 Non sp3 PF6! octahedral AX6 octahedral 90, 180 non sp3d2 BF2Cl triangular planar AX3 triangular planar 120 polar sp2 HCN linear AX2 linear 180 polar sp ICl5 octahedral AX5E square pyramidal ~90, ~180 polar sp3d2 SnBr2 triangular planar AX2E V-shaped ~120 polar sp2 I 3! triangular bipyramidal AX2E3 linear 180 non sp3d XeOF2 triangular bipyramidal AX3E2 T-shape ~90, ~180 polar sp3d SeCl6 octahedral AX6 octahedral 90, 180 non sp3d2 CO2 linear AX2 linear 180 non sp NF3 tetrahedral AX3E triangular pyramidal ~109.5 Polar sp3 COS linear AX2 linear 180 polar sp SiCl2H2 tetrahedral AX4 tetrahedral ~109.5 polar sp3 SOCl2 tetrahedral AX3E triangular pyramidal ~109.5 Polar sp3 PO43! tetrahedral AX4 tetrahedral ~109.5 Non sp3 NOF triangular planar AX2E V-shaped ~120 polar sp2 BrF4+ triangular bipyramidal AX4E sawhorse ~90, ~180, ~120 polar sp3d ClNO2 triangular planar AX3 triangular planar ~120 polar sp2 BeH2 linear AX2 linear 180 Non sp SO32! tetrahedral AX3E triangular pyramidal ~109.5 Polar sp3 ICl4! octahedral AX4E2 square planar 90, 180 Non sp3d2 5. Highest energy: V-shaped (two 90 degree lone pair-lone pair repulsions) > right angled (one 90 degree lone pair-lone pair repulsion) > linear (no 90 degree lone pairlone pair repulsions). Lowest energy is most stable. 6. N2O, B.Order NN bond = 2.5, B. order NO bond = 1.5, NN bond length = 116 pm, NN bond energy = 682 kJ@mol!1 HNO3 B. Order OH bond = 1, B. order for NO bond (O bonded to H) = 1, B. Order other two NO bonds = 1.5 CO32! B. order for each CO bond = 4/3 7. H3C!CO-CN, C1(sp3), C2(sp2), C3(sp), and N(sp) H2N!CH!NH, N1(sp3), C(sp2) and N2(sp2) 8. (a) -1039 kJ@mol!1 (b) 627 kJ@mol!1, % error = 0.6 % 10. H2Se: H(1s1), Se([Ar]3d104s24p4 (2 unpaired 4p electrons for bonding). SnCl2: Cl ([Ne]3s23p5, Sn([Kr] 4d105s25p2), (2 unpaired 5p electrons for bonding) ideal bond angle for both molecules is 90 degrees. 11. molecule #electron groups electron group geometry # hybrid A.O.’s formed hybrid orbital type hybrid orbital geometry bond angles BeH2 2 linear 2 sp linear 180 AlBr3 3 triangular planar 3 sp2 triangular planar 120 CH3Cl 4 tetrahedral 4 sp3 tetrahedral 109.5 NF3 4 tetrahedral 4 sp3 tetrahedral 109.5 SF4 5 triangular bipyramidal 5 sp3d triangular bipyramidal 90,180 120 XeF2 5 triangular bipyramidal 5 sp3d triangular bipyramidal 90,180 120 XeF4 6 octahedral 6 sp3d2 octahedral 90,180 H2CO 3 triangular planar 3 sp2 triangular planar 120 HCN 2 linear 2 sp linear 180 12. A bonding molecular orbital places a high electron charge density between the two nuclei. This reduces the repulsions between the positively charged nuclei, lowering the energy and increasing the stability of the molecule. An anti-bonding molecular orbital places a low electron charge density between the two nuclei. The repulsions between the nuclei increase because they are poorly shielded from each other, increasing the energy and decreasing the stability of the molecule. 13. π M.O. σ M.O. electron density concentrated above and below internuclear axis ( 2 banana shaped clouds) formed from constructive interference (overlap) of parallel np orbitals. Overlap occurs in 2 locations. electron density concentrated along the internuclear axis. Overlap occurs in one location. 14. In normal π bonding, only two 2pz orbitals overlap to form π M.O.’s while in delocalized π bonding, there is continuous overlap of 2pZ orbitals from an unbroken chain of 3 or more atoms forming an equal number of delocalized π M.O.’s. 15. H2! He2+ O2 N2+ C22! Ne2+ Na2 Mg2 P2 CO NO BN 16. VSEPR shape: triangular planar about each carbon giving benzene its flat hexagonal shape, all C’s sp2 hybridized. Each carbon has three unpaired sp2 electrons for σ bonding and one 2p electron for π bonding. Three delocalized π bonding MO’s and three delocalized π antibonding MO’s formed from six 2p AO’s. 17. NO3!, O3, and C4H6 18. Non-bonding delocalized Π molecular orbitals are orbitals whose energy is the same as the 2pz orbitals from which they are formed and hence do not contribute to bonding. One non!bonding Π molecular orbital is formed. (σ1s)2(σ1s*)1 bond order = ½ paramagnetic (σ1s)2(σ1s*)1 bond order = ½ paramagnetic (σ2s)2(σ2s*)2(σ2p)2 (π2p)2(π2p)2 (π2p*)1(π2p*)1 bond order=2 paramagnetic (σ2s)2(σ2s*)2 (π2p)2(π2p)2(σ2p)1 bond order = 2.5 paramagnetic (σ2s)2(σ2s*)2 (π2p)2(π2p)2(σ2p)2 bond order = 3.0 diamagnetic (σ2s)2(σ2s*)2 (σ2p)2 (π2p)2(π2p)2 (π2p*)2(π2p*)2(σ2p*)1 bond order = ½ paramagnetic (σ3s)2 bond order = 1 diamagnetic (σ3s)2(σ3s*)2 bond order = 0 diamagnetic and unstable (σ3s)2(σ3s*)2 (π3p)2(π3p)2(σ3p)2 bond order = 3 diamagnetic (σ2s)2(σ2s*)2 (π2p)2(π2p)2(σ2p)2 bond order = 3.0 diamagnetic (σ2s)2(σ2s*)2 (π2p)2(π2p)2(σ2p)2 (π2p*)1 bond order = 2.5 paramagnetic (σ2s)2(σ2s*)2 (π2p)2(π2p)2 bond order = 2.0 diamagnetic Developed by: Dr. Chris Flinn