* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download bio20 9.2 - Stirling School
Survey
Document related concepts
Transcript
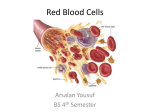
Gas Exchange and Transport Section 9.2 Dalton’s Law of Partial Pressure Each gas in a mixture exerts its own pressure or partial pressure. Partial Pressure Oxygen has its highest partial pressure in the atmosphere. Gasses move from areas of high concentration to areas of low concentration. Therefore, oxygen moves from the atmosphere and diffuses in the lungs. Arteries Capillaries and Veins Picture here Arteries Carry oxygenated blood away from the heart. Veins Carry deoxynated blood back to the heart Capillaries Connect arteries and veins The site of gas exchange Partial Pressure cont. Carbon dioxide has its highest pressure in the tissues of the body. Therefore, carbon dioxide will diffuse out of the tissues. Oxygen Transport Oxygen moves from atmosphere, to the the alveoli, to the blood and dissolves in blood plasma. Plasma: Colorless fluid part of the blood Hemoglobin The Oxygen carrying molecule. Contains iron that binds with oxygen. Oxyhemoglobin: Hemoglobin bonded to oxygen. Hemoglobin drastically increases the bloods capacity to carry oxygen. (70 times greater) Breakdown of Oxyhemoglobin Ultimately, oxygen must be released in order for uptake by the cells. Partial pressure dictates the dissociation of the oxyhemoglobin molecule. At 5.3 kpa dissociation occurs. So What? Important because this is the partial pressure at the capillaries. Oxygen is made available at the site of gas exchange. Nice adaptation eh? Venous Blood Is the blood returning to the heart void of oxygen? No! 70% of the hemoglobin still carry oxygen at their binding sites. Carbon Dioxide Transport Carbon D. is 20 times more soluble in the blood than oxygen. 9% of Caron D. is carried in the blood 27% combines with hemoglobin 64% combines with water to form carbonic acid Formation of Carbonic Acid Carbonic Anhydrase An enzyme Speeds up chemical rxn’s Carbonic Anhydrase speeds the conversion to carbonic acid by 250 times. Carbonic Acid This allows for a rapid way to deal with build up of Carbon D. in the blood plasma The rxn also maintains a low partial pressure of Carbon D. in the blood (carbon d. continues to diffuse into blood.) Problem Carbonic acid will lower the pH of the blood. If we alter the pH of the blood too much the consequences could be catastrophic. Solution Hemoglobin can work as a buffer. Carbonic acid dissociates to bicarbonate ions and hydrogen ions. Dissociation of Carbonic acid Hemoglobin Buffer Hydrogen ions help dislodge oxygen from hemoglobin. Hemoglobin bind with hydrogen ions, thus, removing H ions from the solution. (raises pH or buffers the soln.) Oxygen that has been removed is now free for exchange at capillaries. At lungs, oxygen dislodges hydrogen ions from hemoglobin. Free Hydrogen ions and bicarbonate combine to form water and Carbon D. Highly concentrated Carbon D. diffuses out of blood into alveoli, and expelled through exhalation. At Lungs Maintaining Gas Levels Chemical receptors are commonly employed to ensure carbon d. and oxygen levels are maintained at proper levels. Maintenance of Carbon D. Levels (Example) Exercise leads to a build up of carbon d. Chemical receptors are triggered in the brainstem Causes muscles that control breathing rate to operate at increased levels Carbon D. is flushed from the body Summary Summary