* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fresnel Lens
Hall effect wikipedia , lookup
Metamaterial cloaking wikipedia , lookup
Nanochemistry wikipedia , lookup
Microelectromechanical systems wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
History of metamaterials wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
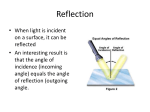
Fresnel Lens • Seen in lighthousesused to form a concentrated beam of light. Fresnel Lens at work • Fresnel lens melting brick • International Automated Systems Fresnel system Reflection • When light is incident on a surface, it can be reflected • An interesting result is that the angle of incidence (incoming angle) equals the angle of reflection (outgoing angle. Reflection from a curved surface • When the surface doing the reflecting is curved, the light can be brought to a focus. • The curved surface can be parabolic or spherical. • Spherical surfaces are cheaper and easier to construct. Power towers • Use many collectors and focus the light to a central point. • Achieves high temperatures and high power density. • Each individual collector is called a heliostat • Must be able to track the sun and focus light on the main tower How they work • • • • • • Light is collected at the central tower, which is about 300 feet tall. There are on the order of 2000 heliostats. Used to heat water and generate steam Steam drives a turbine which generates electricity Often include auxiliary energy storage to continue to produce electricity in the absence of sunlight More costly to construct and operate than coal fired plants. Good candidates for cogenerationwaste steam could be used for space heating Solar troughs • A parabolic shaped trough collects the light and focuses it onto a receiver. • The receiver has a fluid running through it which carries the heat to a central location where it drives a steam turbine • May have more than a hundred separate troughs at such a facility Trough Pictures Direct Conversion of sunlight to energy: Photo-voltaics • Photoelectric effect: • When electromagnetic energy impinges upon a metal surface, electrons are emitted from the surface. • Hertz is often credited with first noticing it (because he published his findings) in 1887 but it was seen by Becquerel In 1839 and Smith in 1873. Photoelectric effect • The effect was a puzzle • The theory of light as a wave did not explain the photoelectric effect • Great example of the scientific method in action. – Up until this point, all the observations of light were consistent with the hypothesis that light was a wave. – Now there were new observations could not be explained by this hypothesis – The challenge became how to refine the existing theory of light as a wave to account for the photoelectric effect Photoelectric effect explained • Einstein in 1905 explained the photoelectric effect by assuming light was made of discrete packets of energy, called photons. • Not a new idea, he was building upon an idea proposed by Planck, that light came in discrete packets. (in fact, Newton proposed a particle like explanation of light centuries earlier). The problem for Planck was his discrete packets were in conflict with the wave like behavior of light. Photoelectric effect explained • But now, a behavior of light was observed that fit Planck’s energy packet idea. • So electromagnetic radiation appears to behave as if it is both a wave and a particle. • In fact, you can think of light as discrete wave packets-packets of waves which, depending upon the measurement you make, sometimes exhibit particle behavior and sometimes exhibit wave behavior. • Einstein won the Nobel prize for his explanation of the photoelectric effect. Semi conductors • Devices which have conductive properties in between a conductor and an insulator. • Normally, the outer (valence) electrons are tightly bound to the nucleus and cannot move. • If one or all of them could be freed up, then the material can conduct electricity • Silicon is an example of a semi-conductor. Silicon • • • • Element 14 in the periodic table Very common element (sand, glass composed of it) 8th most common element in the universe Its 4 outer valence electrons are normal tightly bound in the crystal structure. • However, when exposed to light, the outer electrons can break free via the photoelectric effect and conduct electricity. • For silicon, the maximum wavelength to produce the photoelectric effect is 1.12 microns. 77% of sunlight is at wavelengths lower than this. But its not quite this simple • You also need to produce a voltage within the silicon to drive the current. • So the silicon must be combined with another material. This process is called doping. • 2 types of doping: P and N – If you replace one of the silicon atoms in the crystal lattice with a material that has 5 valence electrons, only 4 are need to bond to the lattice structure, so one remains free. The doped semi conductor has an excess of electrons and is called an N type semiconductor. – Doping elements can be arsenic, antimony or phosphorus. P-types • If you dope with an element with only 3 valence electrons, there is a vacancy, or hole left where the 4th electron should be. • If the hole becomes occupied by an electron from a neighbor atom, the hole moves through the semiconductor. This acts like a current with positive charge flowing through the semi conductor, so it appears to have a net positive charge • Called a P-type semiconductor. • Doping elements could be boron, aluminum, or indium Creating the solar cell • To create the solar cell, bring a p-type silicon into contact with an n-type silicon. • The interface is called a p-n junction. • Electrons will diffuse from the n material to the p material to fill the holes in the p material. This leaves a hole in the n material. • So the n-material ends up with an excess positive charge and the p material ends up with an excess negative charge. • This creates an electric field across the junction. Current in the solar cell • Any free electrons in the junction will move towards the n –type material and any holes will move toward the p -type material . • Now sunlight will cause the photoelectric effect to occur in the junction. Thus free electrons and holes are created in the junction and will move as described above. • Current flows!