* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Environmental Chemistry
Water testing wikipedia , lookup
Electrochemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Water splitting wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Water pollution wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrolysis of water wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
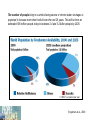
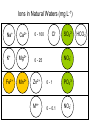
Environmental Chemistry Chapter 10: The Chemistry of Natural Waters Copyright © 2012 by DBS Contents • • • Natural Waters Oxidation and Reduction Chemistry in Natural Waters Acid-Base Chemistry in Natural Waters Natural Waters The Blue Marble 0.001 % water vapor 71 % liquid water The Blue Marble is a famous photograph of the Earth taken on 7 December 1972 by the crew of the Apollo 17 spacecraft at a distance of about 29,000 km or about 18,000 miles. It is one of the most widely distributed photographic images in existence. The image is one of the few to show a fully lit Earth, as the astronauts had the Sun behind them when they took the image. To the astronauts, Earth had the appearance of a child's glass marble (hence the name). Natural Waters Compartments • Atmosphere • Land • Groundwater • Rivers lakes • Oceans Water Cycle Source: http://www.nasa.gov/vision/earth/environment/warm_wetworld.html Where Does Potable (fit for consumption) Drinking Water Come From? Sources Less than one third of salt-free water is liquid Surface water: from lakes, rivers, reservoirs (< 0.01 % of total) Ground water: pumped from wells drilled into underground aquifers (0.3 %) Natural Waters Uses of Water World • 70% irrigation (agriculture) • 20 % Industry • 10% residences World Resources 1998-99 The number of people living in countries facing severe or chronic water shortages is projected to increase more than fourfold over the next 25 years. This will be from an estimated 505 million people today to between 2.4 and 3.2 billion people by 2025. Sources < 1000 m3 per person per year Engelman et al., 2000 Access to Water Access to Water Uneven distribution of water Region Total Renewable Water Resources (km3 yr-1) Total Water Withdrawals (m3 yr-1) Per Capita (m3 person-1) Average % of Renewable Resources Average % Used by Agriculture Average % Used by Industry World 43,249 3,414,000 650 - 71 20 Asia 11,321 1,516,247 1,028 29 79 10 Europe 6,590 367,449 503 9 25 48 518 303,977 754 423 80 5 4,850 512,440 1,720 14 27 58 Middle East/N. Africa N. America Subject to contamination Using water at a rate faster than it can be supplied (>100 due to use of sea water) Natural Waters Role of Water in the Environment • Water is an important constituent in our body and our survival depends on natural waters – transports substances into, within, and out of living organisms – distributes soluble substances (e.g. pesticides, lead, mercury) – reduces concentrations via dilution and dispersion e.g. rainwater carries substances (e.g. acids) down to earth’s surface, washes out (cleanses) the air but pollutes waterways Transport medium volumes, residence times, Water Cycle fluxes Liquid Medium Largest reservoir – oceans τ = 40,000 yr Natural Waters Representations of Water Region of partial negative charge Regions of partial positive charge Polarity Partial charges result from bond polarization A difference in the electronegativities of the atoms in a bond creates a polar bond A polar covalent bond is a covalent bond in which the electrons are not equally shared, but rather displaced toward the more electronegative atom Natural Waters H-Bonding Polarized bonds allow hydrogen bonding to occur • • • A hydrogen bond is an electrostatic attraction between an atom bearing a partial positive charge in one molecule and an atom bearing a partial negative charge in a neighboring molecule The H atom must be bonded to an O, N, or F atom Hydrogen bonds typically are only about one-tenth as strong as the covalent bonds that connect atoms together within molecules H–bonds are intermolecular bonds Covalent bonds are intramolecular bonds Natural Waters Unique Properties • • • • • • • • Water shrinks on melting (ice floats on water) Unusually high melting point Unusually high boiling point Unusually high surface tension Unusually high viscosity Unusually high heat of vaporization Unusually high specific heat capacity And more… Natural Waters Ice shrinks on melting as 15% H-bonds are lost A certain mass of ice occupies more space than the same mass of water Natural Waters Aqueous Chemistry • Organic content of water is dominated by redox chemistry (pE) • Inorganic content of water (dissolved ions) is controlled by acid-base and solubility phenomena (pH-pK) End • Review Redox Chemistry in Natural Waters When the muddy sediments at the bottom of a river or lake are disturbed, two things are noticed. First, there is a smell of rotten eggs, indicating the presence of hydrogen sulfide. Secondly, the color of the sediment may change from a redbrown at the surface to a dark brown-black below. Both of these observations are linked to a lack of oxygen. The hydrogen sulfide will have come from the metabolism of sulfate by sulfatereducing bacteria, the dark coloration is due to the presence of metal sulfides in the sediment which have been formed from this hydrogen sulfide Redox Chemistry in Natural Waters Most important oxidizing agent is dissolved O2 (atmospheric) Acidic solution O2 + 4H+ + 4e- → 2H2O O2 is reduced from 0 to -2 state in H2O or OH− Basic solution O2 + 2H2O + 4e- → 4OH− Concentration of O2 in water is low (10 ppm average), governed by Henry’s law: O2(g) ⇌ O2(aq) KH = [O2 (aq)] PO2 At 25 °C, KH = 1.3 x10-3 mol L-1 atm-1 Dissolved O2 influences chemical speciation of elements in natural and polluted waters Question P9-1: Confirm by calculation the value of 8.7 mg L-1 for the solubility of oxygen in water at 25 °C At 25 °C, KH = 1.3 x10-3 mol L-1 atm-1 KH = [O2 (aq)] / PO2 [O2 (aq)] = KH x PO2 [O2 (aq)] = (1.3 x10-3 mol L-1 atm-1 ) x 0.21 atm = 2.7 x 10-4 mol L-1 [O2 (aq)] = 2.7 x 10-4 mol L-1 x 32.00 g mol-1 = 8.7x 10-3 g L-1 = 8.7 mg L-1 = 8.7 ppm Question P9-2: Given the solubility cited above for O2 at O °C, calculate the value of KH at this temperature At O °C, [O2 (aq)] = 14.7 ppm Since the equilibrium constant uses moles L-1 we must convert: 14.7 g O2 x 1 mol O2 x 1000 g H2O = 4.59 x 10-4 mols L-1 106 g H2O 32.0 g O2 1 L H2O KH = [O2 (aq)] / PO2 KH = 4.59 x 10-4 mols L-1 / 0.21 = 2.2 x 10-3 mols L-1 atm-1 Depletion of DO • • • • Temperature (inc) Pressure (dec) Salts (inc) Organic matter (inc) Dissolved O2 decreases with increasing temperature Redox Chemistry in Natural Waters Oxygen Demand • The most common substance oxidized by DO in water is organic matter (plant debris, dead animals etc.) 0 to -2 CH2O(aq) + O2(aq) → CO2(g) + H2O(aq) 0 to +4 • • • Similarly DO is consumed by NH3 and NH4+ in the nitrification process Water in streams and rivers are constantly replenished with oxygen Stagnant water and deep lakes can have depleted oxygen Redox Chemistry in Natural Waters Oxygen Demand Half reactions Oxidation: Reduction: CH2O + H2O → CO2 + 4e- + 4H+ 4H+ + O2 + 4e- → 2H2O CH2O(aq) + O2(aq) → CO2(g) + H2O(aq) In basic conditions? O2 + 4H+ + 4e- 2 H2O React with hydroxide O2 + 4H+ + 4OH- + 4e- 2H2O + 4OHO2 + 4H2O + 4e- 2H2O + 4OHO2 + 2H2O + 4e- 4OH- Same overall Question P9-4: Determine the balanced redox reaction for the oxidation of ammonia to nitrate ion by O2 in alkaline solution (basic) Does this reaction make the water more basic or less? NH3 + O2 NO3- + H2O Using standard redox balancing techniques: NH3 + 2O2 + OH- NO3- + 2H2O The water becomes less basic since OH- is removed Redox Chemistry in Natural Waters Oxygen Demand • Humic acid is a fraction of the complex mixture known as humic substances or Natural Organic Matter (NOM). Humic substances make up a large portion of the dark matter in humus and are complex colloidal supramolecular mixtures that have never been separated into pure components • • • Humic substances have been designated as either humic acid, fulvic acid or humin. Humic substances arise by the microbial degradation of biomolecules (lipids, proteins, carbohydrates, lignin) dispersed in the environment after the death of living cells. A modern structural description regards humic material as a supramolecular structure of relatively small bio-organic molecules Tahquamenon Falls, Upper MI Measures of amount of organics/biological species in water • • • • • Biochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD) Total Organic Carbon (TOC) Dissolved Organic Carbon (DOC) (TOC)-(DOC) = Suspended carbon in water Redox Chemistry in Natural Waters Biological Oxygen Demand • • The capacity of the organic and biological matter in a sample of natural water to consume oxygen, a process usually catalyzed by bacteria, is called BOD Procedure: measure O2 in the stream or lake. Take a sample and store at 25oC for five days and remeasure O2 content. The difference is the BOD – BOD5 corresponds to about 80% of the actual value. It is not practical to measure the BOD for an infinite period of time – Surface waters have a BOD of about 0.7 mg L-1 – significantly lower than the solubility of O2 in water (8.7 mg L-1) – Sewage has BOD of ~100 mg L-1 Redox Chemistry in Natural Waters Chemical Oxygen Demand O2 + 4H+ + 4e- → 2H2O • Dichromate ion, Cr2O72- dissolved in sulfuric acid is a powerful oxidizing agent. It is used as an oxidant to determine COD Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7 H2O • Excess dichromate is added to achieve complete oxidation Back titration with Fe2+ gives the desired endpoint value # moles of O2 consumed = 6/4 x (#moles Cr2O7 consumed) Note: Cr2O72- is a powerful oxidizing agent and can oxidize species that are not usually oxidized by O2 - hence gives an upper limit Question P9-5: A 25 mL sample of river water was titrated with 0.0010 M Na2Cr2O7 and required 8.7 mL to reach the endpoint. What is the COD (mg O2/L)? No. moles Cr2O72- = 0.0010 mol L-1 x (8.7 x 10-3 L) = 8.7 x 10-6 mols No. moles O2 = 1.5 moles Cr2O72- = 1.5 x (8.7 x 10-6 mols) = 1.3 x 10-5 mols O2 1.3 x 10-5 mol x 32.00 g mol-1 = 4.2 x 10-4 g 0.42 mg / 0.025 L = 17 mg L-1 Redox Chemistry in Natural Waters Decomposition of Organic Matter • In the absence of O2 bacteria present in water can induce chemical transformations 2CH2O −bacteria CH4 + CO2 disproportionation 1966-Michigan Swamp Gas Case March 14-20, 1966: Southeastern Michigan. From about 3:50 a.m. on March 14 and for 2-1/2 hours thereafter, Washtenaw County sheriffs and police in neighboring jurisdictions reported disc-shaped objects moving at fantastic speeds and making sharp turns, diving and climbing, and hovering. At one point, four UFOs in straight-line formation were observed. Swamp Gas Mystery Solved Bohannon, Science NOW 24 August 2005: 2 Redox Chemistry in Natural Waters Aerobic vs. Anaerobic Conditions Fe3+ in sediments gets converted into soluble Fe2+ under reductive conditions Fe3+ + eInsoluble Fe(III) Fe2+ Soluble Fe(II) epilimnion Stratification: In the same lake aerobic and anaerobic condition may exist Hypolimnion – anoxic due to O2 consumption by organic matter Redox Chemistry in Natural Waters The pE Scale • Oxidation and reduction are controlled by the concentrations of electrons which are present: pE = - log10[e-] Low pE means electrons are available (reducing environment) High pE means electrons are unavailable (oxidizing environment) pE = E/0.0591 When a significant amount of O2 is dissolved, the reduction of O2 is the dominant reaction determining e- availability: ¼ O2 + H+ + e- ⇌ ½ H2O • Under such circumstances, the pE of the water is related to its acidity and to the partial pressure as follows: pE = 20.75 + log([H+] PO2¼) OR pE = 20.75 – pH + ¼ log(PO2 ) Redox Chemistry in Natural Waters The pE Scale A convenient approach is to use Nernst Equation of electrochemistry E = E0 – (RT/F) (log [products] / [reactants]) …for 1 electron redox process E = E0 - 0.0591(log [products] / [reactants]) where E0 is the standard electrode potential for a one electron reduction One can equate pE to the Electrode Potential E pE = E/0.0591 or pE0= E0/0.0591 E/0.0591 = E0/0.0591- (log [products] / [reactants]) pE = pE0 - (log [products] / [reactants]) Redox Chemistry in Natural Waters The pE Scale ¼ O2 + H+ + e- ⇌ ½ H2O E0 = 1.23 V pE0 = 1.23/0.0591 pE = pE0 - (log [products] / [reactants]) pE = 20.75 - log 1/ [reactants] = 20.75 + log ([reactants]) pE = 20.75 + log([H+] PO2¼) = = 20.75 + log([H+] + log (PO2¼) pE = 20.75 – pH + ¼ log(PO2 ) pE = 20.75 – pH + ¼ log(PO2 ) For a neutral sample of water that is saturated with oxygen from air (PO2 = 0.21 atm) that is free from CO2 (pH = 7) the pE value corresponds to 13.9 …pE value decreases with decrease in O2 and increase in pH Dominant redox equilibrium reaction determines pE of water (O2 may not be dominant redox species!) Question What is the most oxidizing conditions possible in water? PO2 cannot exceed 1, log(1) = 0 pE = 20.75 – pH pE = 20.75 - pH This can be drawn on a pE/pH diagram as a boundary line, When pE > 20.75 – pH water will be oxidized pE A similar analysis gives boundary below which water will be reduced pE = - pH pH Redox Chemistry in Natural Waters The pE Scale Example 1/8NO3− + 5/4H+ + e- ⇌ 1/8NH4+ + 3/8H2O E0 = +0.836 V pE0 = E0/0.0591 = 0.836/0.0591 = +14.15 pE = pE0 – log [NH4+]1/8 [NO3-]1/8[H+]5/4) ax = x log a log(1/b) = -log b = 14.15 - 5/4pH -1/8log([NH4+]/[NO3-]) Note: Express the reactions as one electron reduction process ….. Follow the examples given on page 435 Question 9-7: Deduce the equilibrium ratio of concentrations of NH4+ to NO3- at a pH of 6.0 (a) for aerobic water having a pE = +11, and (b) for anaerobic water with pE = -3 pE = 14.15 – (5/4)pH – (1/8)log([NH4+] / [NO3-]) 11 = 14.15 – (5/4) x 6 – (1/8)log([NH4+] / [NO3-]) log([NH4+] / [NO3-]) = -8(4.35) = -34.8 [NH4+] / [NO3-] = 1.6 x 10-35 pE = 14.15 – (5/4)pH – (1/8)log([NH4+] / [NO3-]) -3 = 14.15 – (5/4) x 6 – (1/8)log([NH4+] / [NO3-]) log([NH4+] / [NO3-]) = 8(9.65) = 77.2 [NH4+] / [NO3-] = 1.6 x 1077 Problem 9-7 pH = 6, pE = 11, pH = 6, pE = -3, Redox Chemistry in Natural Waters The pE Scale • • • Ferrous iron (Fe2+) hydroxide is more soluble, than ferric (Fe3+) hydroxide. Fe3+(aq) precipitates as the hydroxide under neutral or basic conditions Fe2+ is readily oxidized to Fe3+ by dissolved oxygen 4Fe2+ + O2 + 2H2O 4[Fe3+ + 3OH4Fe2+ + O2 + 2H2O + 8OH- 4 Fe3+ + 4OH4Fe(OH)3(s)] 4Fe(OH)3(s) Well water often deposits brown, rusty, iron hydroxide when brought to the surface and into contact with oxygen Redox Chemistry in Natural Waters The pE Scale • • • • Oxidation in aquatic environment is controlled by MO’s – biologically mediated Low values of O2 found in reducing environments due to microbial catalyzed fermentation of organic matter In cases of low O2 solubility pE is determined by nitrate or sulfate In the extreme case e- availability determined by methane and carbon dioxide 1/8CO2 + H+ + e- ⇌ 1/8CH4 + H2O pE = 2.87 – pH + 1/8 log(PCO2/PCH4) e.g. PCO2 = PCH4 and pH = 7, pE = -4.1 Highly oxygenated 0.81 V (Oxic) Chemistry (fate and transport) of metals and organics changes depending on E0 Redox Chemistry Reactions and Equilibrium Anaerobic -0.40 V (Anoxic) Redox Chemistry in Natural Waters The pE Scale Fe3+ + e- ⇌ Fe2+ • • For this reaction, pE0 = 13.2 pE = 13.2 + log([Fe3+] / [Fe2+]) NOT pH DEPENDENT! e.g. Ratio of Fe3+ to Fe2+ when pE = -4.1 (reducing) -4.1 = 13.2 + log([Fe3+] / [Fe2+]) log([Fe3+] / [Fe2+]) = -17.3 [Fe3+] / [Fe2+] = 5 x 10-18 • Transition between dominance of one form over the other occurs at [Fe3+] = [Fe2+], pE = 13.2 + log(1) = 13.2 + 0 = 13.2 Redox Chemistry in Natural Waters pE – pH Stability Field Diagrams Zone dominance of various oxidation states pE independent pH independent pE independent • Hydrated Fe2+/Fe3+ ions can only exist under acidic conditions • At higher pH Fe3+ is present as Fe(OH)3. Fe(OH)2 does not precipitate until solution becomes significantly basic • Changes in redox conditions govern whether the iron will be in solution or in the sediments Redox Chemistry in Natural Waters Sulfur Compounds Highly reduced -2 state as found in H2S and DMS (CH3-S-CH3) highly oxidized +6 state as encountered in SO42- Similar to the oxidation in air (recall from acid rain chapter), H2S in water is oxidized by O2 first to SO2 and then to H2SO4 H2S + 2O2 → H2SO4 Question P9-10: (a) Balance the reduction half-reaction that converts SO42- to H2S in acidic conditions (b) Deduce the expression relating pE to pH, the concentration of sulfate ion, and the partial pressure of hydrogen sulfide gas, given that for the half reaction, pE0 = +5.75 (c) Deduce the partial pressure of H2S when the sulfate ion concentration is 10-5 M and the pH is 6.0 for water that is in equilibrium with atmospheric oxygen (a) 10H++ SO42- + 8e- H2S + 4H2O (b) Reduce eqn. to 1 electron: 5/4H++ 1/8SO42- + e- 1/8H2S + 1/2H2O pE = pE0 – log(PH2S1/8 / [SO42-]1/8[H+]5/4) = pE0 – -(5/4)(log[H+]) – (1/8)log(PH2S/[SO42-]) = pE0 – (5/4)pH – (1/8)log(PH2S/[SO42-]) = 5.75 – (5/4)pH – 1/8)log(PH2S/[SO42-]) Question P9-10: (c) Deduce the partial pressure of H2S when the sulfate ion concentration is 10-5 M and the pH is 6.0 for water that is in equilibrium with atmospheric oxygen By rearrangement: -(1/8) log (PH2S/[SO42-]) = pE + pE0 + 5/4 pH For any solution in equilibrium with atmospheric O2, we know that: pE = 20.75 – pH + (1/4) log PO2 And because PO2 = 0.21 and pH = 6, here, pE = 20.75 – 6 + (1/4) log (0.21) = +14.58 We substitute this value into the equation for pE of the H2S/SO42- system above to obtain: Question P9-10: (c) Deduce the partial pressure of H2S when the sulfate ion concentration is 10-5 M and the pH is 6.0 for water that is in equilibrium with atmospheric oxygen -(1/8) log (PH2S/[SO42-]) = 14.58 – pE0 + (5/4) x 6 =14.58 – 5.75 + 7.5 = 16.33 log (PH2S/[SO42-]) = -131 Since [SO42-] = 10-5, then Since PH2S/[SO42-] = 10-131 PH2S = 10-131 x 10-5 = 10-136 atm Thus the partial pressure of H2S under these conditions is absolutely negligible Redox Chemistry in Natural Waters Acid Mine Drainage • • Mines (e.g., coal mines) that contain Ferrite (FeS2) often leach out iron in water as a result of air oxidation Under anaerobic condition FeS2 is insoluble in water, but when comes in contact with air is oxidized from S22- (-1 oxidation state) to sulfate ion (+6): 2S22- + 7O2 + 2H2O → 4SO42- + 4H+ • • Since the sulfate of the Fe2+ is soluble in acidic water, the iron pyrite effectively becomes solubilized The reaction produces large amounts of acid, some of which is consumed in air oxidation (catalyzed by MO’s) to form Fe3+ 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O 4FeS2 + 15O2 + 2H2O → 4Fe3+ + 8SO42- + 4H+ i.e. 2Fe2(SO4)3 + 2H2SO4 Redox Chemistry in Natural Waters Acid Mine Drainage Overall reaction 4FeS2 + 15O2 + 2H2O → 4Fe3+ +8SO42- + 4H+ 2Fe2(SO4)3 + 2H2SO4 In other words the insoluble FeS2 produces soluble iron(III) sulfate and sulfuric acid (Note: acidic conditions are required for the dissolution of Fe2(SO4)3) • • If gets diluted with the natural water Fe2(SO4)3 gets deposited as Fe(OH)3 On the other hand, concentrated acid can also liberate toxic heavy metals from the ores in the mines Fe3+ can also serve as an oxidant for the oxidation of S22S22- + 14Fe3+ + 8H2O → 2SO42- +14Fe2+ + 16H+ The phenomenon of acid drainage is currently of importance in abandoned mines Redox Chemistry in Natural Waters Nitrogen Compounds Highly reduced -3 state as found in NH3 or NH4 and highly oxidized +5 state as encountered in NO3- The equilibrium between the highly reduced and oxidized state is given by NH4+ + 3H2O ⇌ NO3- + 10H+ + 8e- pE-pH Diagram • NH4+ + 3H2O ⇌ NO3- + 10H+ + 8e- • Oxidation is highly pH dependent, disfavored in highly acidic environments • For the diagonal line when [NH4+] = [NO3-] pE • = 14.15 - 5/4pH -1/8log(1) = 14.15 - 5/4pH Nitrite ion (NO2-) dominates in relatively small field Sawyer et al., 1994 End • Review Chemical Aspects pH pH = - log [H+] • H+ usually surrounded by water of hydration, written H3O+ • ‘Master Variable’ – controls parameters e.g. speciation • Ranges 5.5 - 9 Acid-Base Chemistry in Natural Waters The Carbonate System • The CO2-Carbonate System CO2(g) + H2O(aq) ⇌ H2CO3(aq) H2CO3(aq) ⇌ H+ + HCO3- • Calcium carbonate though insoluble, leaches out small amounts of CO32- CaCO3(s) ⇌ Ca2+ + CO32- CO32- + H2O ⇌ HCO3- + OH- Acid-Base Chemistry in Natural Waters Water in Equilibrium with Solid Calcium Carbonate • • Hypothetical body of water in equilibrium with excess CaCO3 Solubility product, Ksp = [Ca2+][CO32-] = 4.6 x 10-9 mols2 L-2 at 25 °C From stoichiometry , Solubility of CaCO3, S = [Ca2+] = [CO32-] = 6.8 x 10-5 mol L-1 • Assumes all other reactions negligible BUT! CO32- reacts as a base: CO32- + H2O ⇌ HCO3- + OH- Kb = [HCO3-][OH-] = 2.1 x 10-4 [CO32-] Since equilibrium lies to RHS with excess carbonate in natural waters (low OH-): CaCO3(s) ⇌ Ca2+ + CO32CO32- + H2O ⇌ HCO3- + OHCaCO3(s) + H2O(aq) ⇌ Ca2+ + HCO3- + OH- K = Kb x Ksp = 9.7 x 10-13 K = [Ca2+][HCO3-][OH-] Acid-Base Chemistry in Natural Waters Water in Equilibrium with Solid Calcium Carbonate • Hypothetical reaction: CaCO3(s) + H2O(aq) ⇌ Ca2+ + HCO3- + OHIs the only process of relevance. K = Kb x Ksp = 9.7 x 10-13 = [Ca2+][HCO3-][OH-] From stoichiometry, S = [Ca2+] = [HCO3-] = [OH-] S3 = 9.7 x 10-13 S = 9.9 x 10-5 mol L-1 >>>> 1 component model Since the carbonate ion reacts with water molecules, CaCO3(s) ⇌ Ca2+ + CO32- equilibrium shifts to RHS Question P9-12: Repeat the calculation of the solubility of calcium carbonate by the approximate single equilibrium method using a realistic wintertime water temperature of 5 °C; at that temperature, Ksp = 8.1 x 10-9 for CaCO3, Ka = 2.8 x 10-11 for HCO3-, and Kw = 2.0 x 10-15. Does the solubility of calcium carbonate increase or decrease with increasing temperature? CaCO3(s) + H2O(aq) ⇌ Ca2+ + HCO3- + OHK = Kb x Ksp = Kw x Ksp = 2.0 x 10-15 x 8.1 x 10-9 = 5.8 x 10-13 Ka 2.8 x 10-11 From stoichiometry, S = [Ca2+] = [HCO3-] = [OH-] S3 = 5.8 x 10-13 S = 8.3 x 10-5 mol L-1 i.e. Solubility of CaCO3 increases with temperature Question P9-13: Consider a body of water in equilibrium with calcium sulfate, CaSO4, for which Ksp = 3.0 x 10-5 at 25 °C. Calculate the solubility of calcium sulfate in water assuming that other reactions are negligible. From your answer, calculate the fraction of the sulfate ion that reacts with water to form bisulfate ion: SO42- + H2O ⇌ HSO4- + OHCaSO4(s) ⇌ Ca2+ + SO42- Ka = 1.2 x 10-2 Ksp = [Ca2+][SO42-] If no reaction of sulfate with water, [Ca2+] = [SO42-] S = Solubility of CaSO4 , S2 = [SO42-]2 = 3.0 x 10-5 S = [SO42-] = 5.5 x 10-3 mol L-1 (0.75 g L-1) Question From your answer, calculate the fraction of the sulfate ion that reacts with water to form bisulfate ion: SO42- + H2O ⇌ HSO4- + OH- Ka = 1.2 x 10-2 If reaction of sulfate with water occurs: SO42- + H2O ⇌ HSO4- + OHKb = [HSO4-][OH-] [SO42-] Kb = Kw/Ka = 10-14 / 1.2 x 10-2 = 8.3 x 10-13 Since [HSO4-] = [OH-] and [SO42-] = 5.5 x 10-3 mol L-1 Kb = 8.3 x 10-13 = [HSO4-] 2 / 5.5 x 10-3 mol L-1 [HSO4-] = 6.8 x 10-8 mol L-1 6.8 x 10-8 mol L-1 / 5.5 x 10-3 mol L-1 x 100 = 0.0001% Acid-Base Chemistry in Natural Waters Water in Equilibrium with Both Calcium Carbonate and Atmospheric CO2 • System discussed here is unrealistic since it doesn’t include carbon dioxide and carbonic acid CO2(g) + H2O(aq) ⇌ H2CO3(aq) H2CO3(aq) ⇌ H+ + HCO3CaCO3(s) ⇌ Ca2+ + CO32CO32- + H2O ⇌ HCO3- + OHH+ + OH- ⇌ H2O KH Ka Ksp Kb 1/Kw CaCO3(s) + CO2(g) + H2O(aq) ⇌ Ca2+ + 2HCO3• Giant titration of acid from atmospheric CO2 with base from carbonate ion in rocks K = KspKbKHKa/Kw = 1.5 x 10-6 = [Ca2+][HCO3-]2 PCO2 Acid-Base Chemistry in Natural Waters Water in Equilibrium with Both Calcium Carbonate and Atmospheric CO2 • CaCO3(s) + CO2(g) + H2O(aq) ⇌ Ca2+ + 2HCO3K = KspKbKHKa/Kw = 1.5 x 10-6 = [Ca2+][HCO3-]2 PCO2 If [Ca2+] = S, [HCO3-] = 2S 1.5 x 10-6 = [Ca2+][HCO3-]2 = S (2S)2 PCO2 0.00037 atm S = [CO2] = 5.2 x 10-4 mol L-1 (34 x amount calculated from Henry’s law) S = [Ca2+] = 5.2 x 10-4 mol L-1 (this is 4x closed system) [HCO3-] = 2S = 1.0 x 10-3 mol L-1 Acid-base reaction increase the solubility of both the gas and he solid – water that contains CO2 more readily dissolves calcium carbonate Acid-Base Chemistry in Natural Waters Water in Equilibrium with Both Calcium Carbonate and Atmospheric CO2 • CO32-, H+ and OH- can be derived Ksp = [Ca2+][CO32-] [CO32-] = 8.8 x 10-6 mol L-1 Kb = [HCO3-][OH-] [CO32-] [OH-] = 1.8 x 10-6 mol L-1 Kw = [H+][OH-] [H+] = 5.6 x 10-9 mol L-1 Conclude natural water at 25 °C with a pH determined by saturation with CO2 and CaCO3 should be alkaline (pH = 8.3) Actual value of calcereous waters is around 7-9 Acid-Base Chemistry in Natural Waters Question P9-15: In waters subject to acid rain the pH is not determined by the CO2-carbonate system but rather by the strong acid from the precipitation. Assuming that equilibrium with atmospheric carbon dioxide is in effect, calculate the concentration of HCO3- in natural waters with pH = 6 and 4 at 25 °C, for which KH = 3.4 x 10-2 and Ka for H2CO3 = 4.5 x 10-7 HCO3- + H2O ⇌ H2CO3 + OHKb = [H2CO3][OH-] = 2.2 x 10-8 [HCO3-] [H2CO3] = 1.2 x 10-5 So (from Henry’s law, KH = [H2CO3]/PCO2 = 3.4 x 10-2 / 0.00037 [HCO3-] = [H2CO3][OH-] / 2.2 x 10-8 [HCO3-] = 5.5 x 102[OH-] Now at pH = 6, pOH = 8, [OH-] = 10-8 [HCO3-] = 5.5 x 102 x 10-8 = 5.5 x 10-6 Now at pH = 4, pOH = 10, [OH-] = 10-10 [HCO3-] = 5.5 x 102 x 10-10 = 5.5 x 10-8 End • Review Ions in Natural Waters and Drinking Waters The Abundant Ions • • Bicarbonate, HCO3Calcium, Ca2+ • • • • • • Magnesium ion, Mg2+ Sulfate, SO42Chloride, ClSodium, Na+ Fluoride, FPotassium, K+ most abundant ions in unpolluted waters Found in 2:1 ratio Ions in Natural Waters (mg L-1) Na+ Ca2+ K+ Mg2+ Fe2+ Mn2+ 0 - 100 Cl- NO3- 0 - 25 Zn2+ Mn+ SO42- 0-1 PO43- 0 – 0.1 NO2- HCO3- Ions in Natural Waters and Drinking Waters Ions in Natural Waters + Drinking Waters Fluoride • • • • • • [F-] < 0.01 ppm – 20 ppm Natural source is fluorapatite, Ca5(PO4)3F Fluorosilicic acid H2SiF6 is added to increase [F] to 1 ppm Form of mass medication…reduces cavities? Higher concentrations causes mottling of teeth Maximum contaminant level (MCL) level is 4 ppm http://en.epochtimes.com/news/5-5-16/28765.html Question What is 1 ppm F- in mols L-1? 1 ppm F- = 1 mg L-1 = 10-3 g L x 1 mol = 5 x 10-5 mol L-1 19.00 g Ions in Natural Waters + Drinking Waters Alkalinity • • • Dissolution of limestone and other minerals produces alkalinity e.g. CaCO3 ⇌ Ca2+ + CO32CO32- + H2O ⇌ HCO3- + OHWater supply with high total alkalinity is resistant to pH change Two samples with identical pH but different alkalinity behave differently on addition of acid – Different capacity to neutralize acid Mineral Composition Calcite CaCO3 Magnesite MgCO3 Dolomite CaCO3.MgCO3 Brucite Mg(OH)2 Ions in Natural Waters + Drinking Waters Alkalinity • Measurement of the buffer capacity (resistance to pH change) also called acid neutralizing capacity (ANC) / total alkalinity e.g. Carbonate neutralization reaction CO32- + H+ ⇌ HCO3Bicarbonate neutralization reaction HCO3- + H+ ⇌ H2O.CO2 ⇌ H2O + CO2 Lime (Ca(OH)2 and soda ash (Na2CO3) used to neutralize industrial wastewater Hydroxide neutralization reaction H+ + OH- ⇌ H2O • • Units are mg L-1 CaCO3 (regardless of species) Measure by titration the quantity of acid or base needed to change the pH to 4.5 (methyl orange end-point) CaCO3 + H2SO4 → H2CO3 + CaSO4 • If pH < 4.5 there is no acid neutralizing capacity i.e. no need to measure alkalinity Question What is the total alkalinity in mg L-1 and mmol L-1 CaCO3 for a sample requiring 21.25 mL of 0.0100 M H2SO4? 0.02125 L x 0.0100 mol L-1 = 2.125 x 10-4 mol H2SO4 Mole ratio is 1:1 2.125 x 10-4 moles H2SO4 = 2.125 x 10-4 moles CaCO3 2.125 x 10-4 mol CaCO3 x 100.09 g / mol = 2.13 x 10-2 g = 21.3 mg 21.3 mg CaCO3 = 213 mg CaCO3 L-1 0.100 L (= 3.28 mmol L-1) Ions in Natural Waters + Drinking Waters Hardness Index Hard water contains high concentrations of dissolved calcium and magnesium ions. Soft water contains few of these dissolved ions. Hardness = [Ca2+] + [Mg2+] Counter ions of alkalinity ions Alkalinity is a good indicator of hardness and vice-versa A pipe with hard-water scale build up Ions in Natural Waters + Drinking Waters Hardness Index • • Because calcium ions, Ca2+, are generally the largest contributors to hard water, hardness is usually expressed in parts per million of calcium carbonate (CaCO3) by mass It specifies the mass of solid CaCO3 that could be formed from the Ca2+ in solution, provided sufficient CO32- ions were also present: Ca2+(aq) + CO32–(aq) → CaCO3(s) • A hardness of 10 ppm indicates that 10 mg of CaCO3 could be formed from the Ca2+ ions present in 1 L of water Ions in Natural Waters + Drinking Waters Hardness Index • • Deposition of white solid CaCO3 or MgCO3 when water is heated – ‘furring-up blocks pipes and lowers efficiency of industrial processes Formation of scum (insoluble ppt) with soap and water Ca2+(aq) + H3C-(CH2)10-COO-(aq) • [H3C-(CH2)10-COO-]2Ca2+(s) – detergent action is blocked Staining (due to transition metals) A pipe with hard-water scale build up Types of Hardness • Solid deposit = carbonate hardness or temporary hardness CaCO3(s) + H2CO3 ⇌ Ca2+ + HCO3- (removed via boiling) – Causes deposit in pipes and scales in boilers – Temporary hard water has to be softened before it enters the boiler, hot-water tank, or a cooling system • No solid = non-carbonate or permanent hardness – Amount of metal ions that can not be removed by boiling Total hardness = temporary hardness + permanent hardness Ions in Natural Waters + Drinking Waters Hardness Index "According to the U.S. National Academy of Sciences (1977) there have been more than 50 studies, in nine countries, that have indicated an inverse relationship between water hardness and mortality from cardiovascular disease” Source: http://www.mgwater.com Ions in Natural Waters + Drinking Waters Hardness Index Beneficial effects: • Alkalinity lowers solubility of toxic metals • Buffering action of CO32- lessens effects of acidic pollutants • Non-toxic essential nutrients • Reduces heart disease • More pleasant to drink Question What is the value of the hardness index for a 500 mL sample of water that contains 0.0040 g of calcium ion and 0.0012 g of magnesium ion? 0.0040 g Ca x 1 mol / 40.1 g = 0.0001 mol Ca 0.0012 g Mg x 1 mol / 24.3 g Mg = 0.00005 mol Mg Since the volume is 0.5 L: [Ca2+] = 0.0001 / 0.5 = 0.0002 mol L-1 [Mg2+] = 0.00005 / 0.5 = 0.0001 mol L-1 Total hardness = 0.0002 + 0.0001 = 0.0003 mol L-1 0.0003 mol L-1 x 100 g CaCO3 / mol = 30 mg CaCO3 L-1 Ions in Natural Waters + Drinking Waters Aluminum • Solubility of Al in natural waters is small, [Al] ~10-6 M Al(OH)3 ⇌ Al3+ + 3OHe.g. Ksp = 10-33 Ksp = [Al3+][OH-]3 = 10-33 pH = 6, [Al3+] = 10-33/(10-8)3 = 10-9 M pH = 5, [Al3+] = 10-6 M pH = 4, [Al3+] = 10-3 M • • Al is much more soluble in acidified rivers and lakes Principle cation in waters of pH < 4.5 exceeding Ca2+ and Mg2+ – Precipitation of Al(OH)3 on fish gills prevents intake of O2 – Also contributes to forest decline For every 1 pH [Al3+] inc. 103 Question What is the concentration of dissolved aluminum in water with a pH of 5.5? Al(OH)3 ⇌ Al3+ + 3OH- Ksp = 10-33 e.g. Ksp = [Al3+][OH-]3 = 10-33 pH + pOH = 14, at pH = 5.5 pOH = 8.5 pOH = 8.5 = -log10 [OH-] [OH-] = 3.2 x 10-9 pH = 5.5, [Al3+] = 10-33/(3.2 x 10-9)3 = 3 x 10-8 mol L-1 Mass Al = 8.2 x 10-7 g Ions in Natural Waters + Drinking Waters Perchlorate, ClO4• Mostly released as ammonium perchlorate – used as oxidizing agents, rocket fuels, fire works, airbags etc • Most of the release in the southwestern part • No federal limit has been set, although some states have set their limits Source: Logan, 2001 Ions in Natural Waters + Drinking Waters Perchlorate, ClO4• • US drinking water ClO4- levels 5 – 20 ppb No federal standard has been set – proposed 1 ppb standard considered too low by NASA and DOD • High doses – affects hormone production by thyroid gland – Maternal hormone levels vital to fetal brain development • Low doses – unknown • Difficult to remove from water systems