* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 2 MEASUREMENTS AND MOLES
History of molecular theory wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Stoichiometry wikipedia , lookup
Mass spectrometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
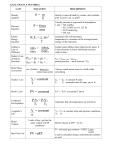
Chapter 2 MEASUREMENTS AND MOLES Metric System We use the SI (System International) unit for all scientific work. A measurement is reported as the numerical multiple of a standard unit. SI Base Unit Quantity Length Mass Time Electric current Temperature Chemical amount Luminous intensity Base Unit Meter (m) Kilogram (kg) Second (s) Ampere (A) Kelvin (K) Mole (mol) Candela (cd) SI prefixes Prefix G M k d c m µ n p Name giga mega kilo deci centi milli micro nano pico Meaning 109 106 103 10-1 10-2 10-3 10-6 10-9 10-12 Extensive and Intensive Properties Extensive properties are properties that depend on the size of the sample example mass and volume. Doubling the sample doubles both mass and volume. Intensive properties are properties that are independent on the size of the sample example density ( a ratio of mass and volume). Another example is temperature. If we draw 10ml of water from a 1L of water the temperature remains the same everytime. Class Practice Find the density of a metal if the water in a graduated cylinder rises from 50ml to 61.5 ml when a piece of the metal of mass 35.55 g is placed in it. A rock of mass 30.6 g that appears to be beryl is found in a forest preserve. When it is placed in a cylinder of water that has been filled to the 60ml mark, the level of the water rises to 78.4 ml. A) Find the density of the rock and decide if it could be beryl. (density of beryl=2.66g/cm3) B) How many cubic cm would 200 g of rock of the same substance occupy? Class Practice On Conversion 1) 678 ml= L 2) 46.5 L = ml 3) 1420 C= K 4) 176µg = kg 5) 16.5 mol= mmol 6) 56L= cm3 7) 465mg= µg 8) 650 nanometers= m. Significant Figures Digits from 1-9 are always significant. Zeros between two other significant digits are always significant One or more additional zeros to the right of both the decimal place and another significant digit are significant. Zeros used solely for spacing the decimal point (placeholders) are not significant. Examples Of Significant Figures 453 kg All non-zero digits are always significant. 5057 L Zeros between 2 sig. dig. are significant. 5.00 Additional zeros to the right of decimal and a significant figures are significant. 0.0071 Placeholders are not significant. Class Practice Report the number of significant figures in a) 50.00 g B) 0.00501 m C) 0.0100 mm D) 350 ml Accuracy And Precision Precision of a measurement refers to how close to one another the repeated measurements are. Accuracy of a series of measurements is the closeness of their average value to the true value. Errors Systematic error are reproducible inaccuracies that are consistently in the same direction. Systematic errors are often due to a problem which persists throughout the entire experiment. Random errors are statistical fluctuations (in either direction) in the measured data due to the precision limitations of the measurement device. Random errors usually result from the experimenter's inability to take the same measurement in exactly the same way to get exact the same number. An accurate measurement is one free of systematic error. A precise measurement is one free of random error. Mole 1 mole is the number of atoms in exactly 12 g of carbon-12. The mass of a carbon-12 atom is 1.992× 10-23 Number of carbon-12 atoms= 12/ 1.992 × 10-23 =6.022 × 10-23 (Avogadro’s Constant) Class Practice A sample of vitamin C is known to contain 2.58 × 10-24 oxygen atoms. How many moles of O atoms are present in the sample? Molar mass Mass of the element per mole of its atom is the molar mass. The molar mass of a molecular compound is the mass of the compound per mole of its molecule. The molar mass of an ionic compound is the mass of the compound per mole of its formula unit. Class Practice Give the molar masses of the following compounds: 1. sodium fluoride 2. potassium hydroxide 3. copper (I) chloride 4. manganese (IV) oxide 5. calcium sulfate 6. magnesium phosphate Average molar mass There are two naturally occurring isotopes of chlorine-35 and chlorine37. The mass of an atom of chlorine35 is 5.807 × 10-23 g and that of an atom of chlorine-37 is 6.139 × 10-23 g . In a typical natural sample of chlorine,75.77% of the sample is chlorine -35 and 24.23% is chlorine-37.What is the average molar mass of chlorine? Empirical formula Empirical formula of a compound is a chemical formula that shows the relative numbers of atoms of each element, using the smallest whole numbers of atoms. Empirical formula for glucose is CH2O tells us that carbon, hydrogen and oxygen are present in the ratio of 1:2:1.The molecular formula for glucose is C6H12O6. Mass Percent Composition To determine the empirical formula of a compound we start by measuring the mass of each element present in the sample. The composition is usually reported as mass percentage composition. Mass%= mass of the element/total mass ×100% Class Practice The analysis of a sample of eucalyptal (an ingredient from eucalyptus tree to cure sore throats) of total mass 3.16 g gave its composition as 2.46 g carbon,0.373 g hydrogen, and 0.329 g oxygen. Determine the mass percentage of carbon in eucalyptol. A compound that helps in the coagulation of blood has the mass percentage composition 76.7% C, 7.02% H, and 16.27%N.Determine the empirical formula of the compound.