* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry Fourth Edition
Survey
Document related concepts
Transcript
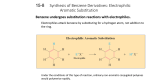
Crown Ethers Crown Ethers structure cyclic polyethers derived from repeating —OCH2CH2— units properties form stable complexes with metal ions applications synthetic reactions involving anions 18-Crown-6 O O O O O O negative charge concentrated in cavity inside the molecule 18-Crown-6 O O O O O O negative charge concentrated in cavity inside the molecule 18-Crown-6 O O O K+ O O O forms stable Lewis acid/Lewis base complex with K+ 18-Crown-6 O O O K+ O O O forms stable Lewis acid/Lewis base complex with K+ Ion-Complexing and Solubility K+F– not soluble in benzene Ion-Complexing and Solubility O O O K+F– O O benzene O add 18-crown-6 Ion-Complexing and Solubility O O O O F– O O K+ O O benzene O O 18-crown-6 complex of K+ dissolves in benzene O O Ion-Complexing and Solubility O O O O O O K+ O O benzene O F– carried into benzene to preserve electroneutrality O O O + F– Application to organic synthesis Complexaton of K+ by 18-crown-6 "solubilizes" salt in benzene Anion of salt is in a relatively unsolvated state in benzene (sometimes referred to as a "naked anion") Unsolvated anion is very reactive Only catalytic quantities of 18-crown-6 are needed Polyether Antibiotics Example KF CH3(CH2)6CH2Br 18-crown-6 benzene CH3(CH2)6CH2F (92%) Polyether Antibiotics Monensin Polyether Antibiotics Monensin Polyether Antibiotics Monensin B Monensin A O O O O O O Na H O HO H O H O O O O O O O Na H O HO H O H O O O