* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture Notes 9 - La Salle University
Survey
Document related concepts
Transcript
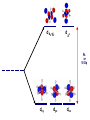
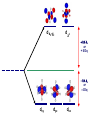
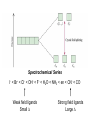
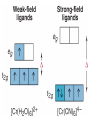
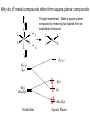
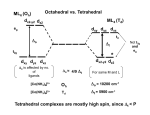
Crystal Field Theory, Electronic Spectra and MO of Coordination Complexes Or why I decided to become an inorganic chemist or Ohhh!!! The Colors!!! Gemstone owe their color from trace transition-metal ions • Corundum mineral, Al2O3: Colorless • Cr Al : Ruby • Mn Al: Amethyst • Fe Al: Topaz • Ti &Co Al: Sapphire • Beryl mineral, Be3 Al 2Si6O18: Colorless • Cr Al : Emerald • Fe Al : Aquamarine d x -y 2 2 d z2 Do or 10 Dq dxy dyz dxz d x -y 2 2 d z2 + 0.6 Do or + 6 Dq - 0.4 Do or - 4 Dq dxy dyz dxz Let’s Look at 4 Co 3+ complexes: Config. [Co(NH3)6]3+ [Co(NH3)5(OH2 Color of Complex Absorbs d6 )]3+ [Co(NH3)5Br]2+ d6 [Co(NH3)5Cl]2+ d6 350-400 400-500 Values are in nm Greater d6 520-570 Splitting 600-700 600-650 570-600 OTHER QUESTIONS So there are two ways to put the electrons Low Spin High Spin Which form for our 4 cobalt(III) complexes? And why the difference between Cl- and Br-? R. Tsuchida (1938) noticed a trend in while looking at a series of Cobalt(III) Complexes. With the general formula : [Co(NH3)5X] look at that! The same ones we just looked at…. He arrived a series which illustrates the effect of ligands on Do (10Dq) He called it: The Spectrochemical Series Tsuchida, R. Bull. Chem. Soc. Jpn. 1938, 13, 388 The Spectrochemical Series Ligand effect on Do : Small Do I- < Br- < S2- < Cl- < NO3- < F- < OH- < H2O < CH3CN < NH3 < en < bpy < phen < NO2- < PPh3 < CN- < CO Large Do Or more simply : X<O<N<C Metals also effect Do : Mn2+ < Ni2+ < Co2+ < Fe2+ < V2+ < Fe3+ < Co3+ < Mn4+ < Mo3+ < Rh3+ < Ru3+ < Pd2+ < Ir3+ < Pt2+ Fe3+ << Ru3+ Ni2+ << Pd2+ Important consequences result!!! Spectrochemical Series I- < Br- < Cl- < OH- < F- < H2O < NH3 < en < CN- < CO Weak field ligands Small D Strong field ligands Large D [Fe(H2O)6]3+ [Ni(H2O)6]2+ [Co(H2O)6]2+ [Zn(H2O)6]2+ [Cu(H2O)6]2+ S=5/2 S=5/2 S=1/2 S=1 S=2 Spectrochemical Series Another important question arises: How does filling electrons into orbitals effect the stability (energy) of the d-orbitals relative to a spherical environment where they are degenerate? We use something called Crystal Field Stabilization Energy (CFSE) to answer these questions For a t2gx egy configuration : CFSE = (-0.4 · x + 0.6 · y)Do So Lets take walk along the d-block…….and calculate the CFSE d1 config. [t2g1]: S=1/2 CFSE = –0.4 Do d2 config. [t2g2]: S=1 CFSE = –0.8 Do d3 config. [t2g3]: S=3/2 CFSE = -1.2 Do BUT WHEN YOU GET TO: d4 THERE ARE TWO OPTIONS!!!!! Low Spin High Spin CFSE = -1.6 Do + P CFSE = -0.6 Do When is one preferred over the other ????? It depends. (P 14,900 cm-1 / e- pair) P = Do both are equally stabilized P > Do high spin (weak field) stabilized NOTE: the text uses the symbol P, for spin pairing energy P < Do low spin (weak field) stabilized P , Spin Pairing Energy is composed of two terms (a) The coulombic repulsion – This repulsion must be overcome when forcing electrons to occupy the same orbital. As 5-d orbitals are more diffuse than 4-d orbitals which are more diffuse than 3-d orbitals, the pairing energy becomes smaller as you go down a period. As a rule 4d and 5d transition metal complexes are generally low spin! (b) The loss of exchange energy – The exchange energy (Hünd’s Rule) is proportional to the number of electrons having parallel spins. The greater this number, the more difficult it becomes to pair electrons. Therefore, d5 (Fe3+ , Mn2+) configurations are most likely to form high spin complexes. Pairing energy for gaseous 3d metal ions M2+ P (cm-1) M3+ P (cm-1) d4 Cr2+ 23,500 Mn3+ 28,000 d5 Mn2+ 25,500 Fe3+ 30,000 d6 Fe2+ 17,600 Co3+ 21,000 d7 Co2+ 22,500 Ni3+ 27,000 Pairing energies in complexes are likely to be 15-30% lower, due to covalency in the metal-ligand bond. These values are on average 22% too high. C. K. Jørgensen’s f and g factors Do = f (ligand) · g (metal) Do in 1000 cm-1 (Kkiesers) g factors f factors 3d5 Mn(II) 8.0 Br - 0.72 3d8 Ni (II) 8.7 SCN - 0.73 3d7 Co(II) 9.0 Cl - 0.78 12.0 N3 - 0.83 F- 0.90 3d3 V(II) 3d5 Fe(III) 14.0 3d3 Cr(III) 17.4 oxalate2- 0.99 3d6 Co(III) 18.2 H2O 1.00 3d9 Cu(II) 9.5 NCS - 1.02 3d4 Cr(II) 9.5 CH3CN 1.22 4d6 Ru(II) 20.0 pyridine 1.23 NH3 1.25 3d3 Mn(IV) 23.0 3d3 Mo(III) 24.6 en (ethylenediamine) 1.28 4d6 Rh(III) 27.0 bipy (2,2’-bipyridine) 1.33 4d3 Tc(IV) 30.0 5d6 Ir(III) 32.0 5d6 Pt(IV) 36.0 Phen (1:10-phenanthroline) CN - 1.34 1.70 Note: Rh3+ and Ir3+ are a lot different than Co3+ g3d < g4d ≤ g5d EXAMPLE: Calculate the Do (10Dq) for [Rh(OH2)6]3+ in cm-1 and nm. for [Rh(pyr)3Cl3] Tetrahedral Coordination Dt = 4/9Do All tetrahedral compounds are High Spin Why do d8 metal compounds often form square planar compounds z Thought experiment: Make a square planar compound by removing two ligands from an octahedral compound L L L M L y L L dx2-y2 dz2 x L L M L L dx2-y2 dxy dxy dxz,dyz dz2 dxz,dyz Octahedral Square Planar dx2-y2 dx2-y2 dz2 dxy dxz,dyz dxy dxy dxz,dyz dx2-y2 dz 2 H2O H2O Ni dxz,dyz Tetrahedral Octahedral OH2 dz 2 2 OH2 Cl OH2 H2O Octahedral Coordination number =6 Ni(II) d8 S = 1 Cl Square Planar 2- N N Cl C C Ni 2- Ni Cl Tetrahedral (CN=4) C N C N Square Planar (CN=4) Ni(II) d8 S =1 Ni(II) d8 S = 0 The Energy Levels of d-orbitals in Crystal Fields of Different Symmetries