* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Epidemiology, Pathophysiology, and Diagnosis of Pulmonary
Survey
Document related concepts
Transcript
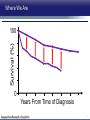
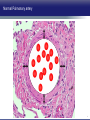
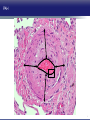
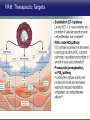
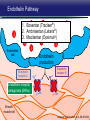
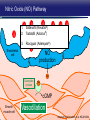
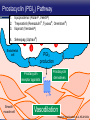
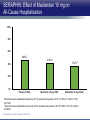
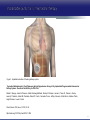
Updates in PAH and what's on the Horizon Abubakr A Bajwa. MD, FCCP Associate Professor of Medicine Associate Chair of Operation, Department of Medicine Division Chief, Pulmonary, Critical Care and Sleep Medicine Medical Director Respiratory Therapy Director Pulmonary Hypertension and Interstitial Lung Disease Clinic University of Florida College of Medicine/Jacksonville Mark Rumbak .MD Professor of Medicine, Pulmonary and Critical Care Medicine Division Director, Pulmonary, Critical Care and Sleep Medicine Director, Bronchoscopy and Interventional Pulmonology Section Chief, Tampa General Hospital Disclosure Consultant: United Therapeutics Grants: UT, James and Esther King Biomedical Foundation, UF Research: UT, Gilead, Actelion, Bayer, AZ, GSK, Intermune, BI 2 Objectives Learn about how your physician determines the right treatment for you Learn about the goals of your PAH treatment Learn about new treatment targets being studied in patients with PAH Gain knowledge on the advances of clinical trials in PAH Understand the benefits and risks of participating in clinical trials 3 Where We Began • • Survival (%) 100 No treatment available Less than 50% of people with PAH lived more than 3 years from the time of diagnosis 0 Years From Time of Diagnosis Adapted from D’Alonzo GE Ann Intern Med 1991 4 Where We Are Survival (%) 100 0 Years From Time of Diagnosis Adapted from Benza RL Chest 2012 5 Normal Pulmonary artery 6 PAH 7 PAH: Therapeutic Targets 8 Timeline of PAH Drug Development Treprostinil intravenous Ambrisentan Room temperature stable epoprostenol Treprostinil subcutaneous Macitentan Riociguat Sildenafil Bosentan Tadalafil Epoprostenol 1995 2001 2002 2004 2005 Iloprost Courtesy of Dr. Harold I. Palevsky 2007 2009 Treprostinil inhaled 2010 2013 Selexipag 2014 2015 Treprostinil oral 9 Endothelin Pathway 1. Bosentan (Tracleer®) 2. Ambrisentan (Letaris®) 3. Macitentan (Opsimut®) Endothelial cell Endothelin production Endothelin receptor A Endothelin receptor B Endothelin receptor antagonists (ERAs) Smooth muscle cell 10 Adapted from Humbert et al. NEJM 2004 Nitric Oxide (NO) Pathway 1. Sildenafil (Revatio®) 2. Tadalafil (Adcirca®) 3. Riociguat (Adempas®) Endothelial cell NO production NO Guanylate cyclase NO cGMP Smooth muscle cell Vasodilation 11 Adapted from Humbert et al. NEJM 2004 Prostacyclin (PGI2) Pathway 1. Epoprostenol (Flolan®, Veletri®) 2. Treprostinil (Remodulin®, Tyvaso®, Orenitram®) 3. Iloprost (Ventavis®) 4. Selexipag (Uptravi®) Endothelial cell PGI2 production Prostacyclin Prostacyclin receptor receptor agonists Smooth muscle cell Prostacyclin derivatives Vasodilation 12 Adapted from Humbert et al. NEJM 2004 Goals of PAH Treatment •Improve survival •Maintain or improve quality of life •Decrease symptoms •Improve exercise capacity •Minimize side-effects of treatment 13 Patients without an Event (%) Advances In Clinical Trials 100 90 80 70 60 50 40 30 12-16 weeks 20 10 0 0 6 12 18 Months 24 30 36 • Hospitalization for worsening PAH • Decreased 6 MWD AND worse symptoms needing additional treatment • Need for continuous prostacyclin treatment • Lung transplant • Death 14 AMBITION: Initial Combination Therapy With Ambrisentan-Tadalafil Compared With Monotherapy Primary Endpoint: Time to First Clinical Failure Event Primary Analysis Set 100 1 year 88.9% 2 year 79.7% Event-Free (%) 75 3 year 67.6% 1 year 75.5% 50 2 year 63.2% 3 year 56.1% 25 HR: 0.502 95% CI: 0.348-0.724 P=0.0002 Combination therapy Pooled monotherapy 0 0 24 48 72 96 120 144 168 192 Time (weeks) Number at Risk: Combination 253 229 186 145 106 71 36 4 Pooled monotherapy 247 209 155 108 77 49 25 5 Galie N, et al. N Engl J Med. 2015;373:834-844 15 GRIPHON: Selexipag for PAH Primary Endpoint: Time to First Event Patients without an event (KM)% 100 80 60 Selexipag 40 Placebo 20 Selexipag vs placebo: Risk reduction 40% HR = 0.60; 99% CI, 0.46-0.78; P<0.0001 0 0 6 12 Selexipag 24 30 36 149 171 88 101 28 40 Months No. at Risk: Placebo 18 582 574 433 455 Sitbon O, et al. N Engl J Med. 2015:373:2522-2533. 347 361 220 246 16 SERAPHIN: Effect of Macitentan 10 mg on All-Cause Hospitalization 100% 80% 60% 46.8% 41.6%* 40% 37.2% † 20% 0% Placebo (n=249) Macitentan 3 mg (n=250) Macitentan 10 mg (n=242) *Risk of all-cause hospitalization reduced by 18.9% compared with placebo. HR: 0.811 (95% CI: 0.623 to 1.057); P=0.1208. † Risk of all-cause hospitalization reduced by 32.3% compared with placebo. HR: 0.677 (95% CI: 0.514 to 0.891); P=0.0005. Channick RN, et al. JACC Heart Fail. 2015;3:1-8. 17 COMPERA Registry: Anticoagulation Effect on Survival in IPAH and APAH IPAH Survival APAH Survival 100% 100% P=0.017 P=0.28 80% Survival of Patients Survival of Patients 80% 60% 40% 20% No anticoagulation Anticoagulation 0% 0 1 2 3 Disease Duration Since IPAH Diagnosis (years) N=168 patients with newly diagnosed IPAH receiving anticoagulation versus matched pairs never receiving anticoagulation. Olsson KM, et al. Circulation. 2014;129:57-65. 60% 40% 20% No anticoagulation Anticoagulation 0% 0 1 2 3 Disease Duration Since APAH Diagnosis (years) N=210 patients with APAH receiving anticoagulation and N=273 patients without anticoagulation. 18 REVEAL: Use of Warfarin in IPAH Survival Through 36 Months 100 P=0.17 81.1 ± 3.6% Survival (%) 90 80 70 77.2 ± 3.7% Started warfarin on study (n=144) Never on warfarin (n=144) 60 Hazard Ratio 95% Confidence Interval P value Unadjusted 1.42 0.86, 2.32 0.17 REVEAL risk score adjusted 1.37 0.84, 2.25 0.21 50 40 0 0 6 12 18 24 30 36 Time From Warfarin Start/Match (months) No. at risk Warfarin Never warfarin 144 144 124 135 119 110 108 110 93 95 83 81 77 67 N=144 patients with IPAH who initiated warfarin while enrolled in REVEAL, compared with 144 matched patients never receiving warfarin. Preston I, et al. Circulation, 2015;132:2403-2411. 19 REVEAL: Survival by 1-year Change in 6MWD 100% 95% 90% 85% 80% 75% 70% 65% 60% 55% 50% Newly Diagnosed Patients Patients with change in 6MWD within 10 percentage points -45 -35 -25 -15 -5 5 15 25 Percent Change in 6MWD 35 45 One-year Survival One-year Survival All Patients 100% 95% 90% 85% 80% 75% 70% 65% 60% 55% 50% Patients with change in 6MWD within 10 percentage points -45 -35 -25 -15 -5 5 15 25 Percent Change in 6MWD 35 45 N=1,798 patients enrolled in REVEAL registry with baseline and 1-year follow-up 6MWD. Farber HW, et al. J Heart Lung Transplant. 2015;34:362-368. 20 Figure 1. Implantation location of the drug delivery system. Treprostinil Administered to Treat Pulmonary Arterial Hypertension Using a Fully Implantable Programmable Intravascular Delivery System : Results of the DelIVery for PAH Trial Robert C. Bourge, Aaron B. Waxman, Mardi Gomberg-Maitland, Shelley M. Shapiro, James H. Tarver III, Dianne L. Zwicke, Jeremy P. Feldman, Murali M. Chakinala, Robert P. Frantz, Fernando Torres, Jeffrey Cerkvenik, Marty Morris, Melissa Thalin, Leigh Peterson, Lewis J. Rubin Chest, Volume 150, Issue 1, 2016, 27–34 http://dx.doi.org/10.1016/j.chest.2015.11.005 21 PAH Therapy: Experimental Therapy Drug Repurposing: Tacrolimus (FK-506) • Mutations in the BMPR2 gene are the most common genetic cause of PAH • Impaired BMPR2 signaling is very common even in PAH patients who do not have a gene mutation • Identified by molecular techniques searching for drugs that activate BMPR2 signaling (“drug repurposing”) JCI 2013 and AJRCCM 2015 23 Drug Repurposing: Tacrolimus (FK-506) • Demonstrated promising effects in PH animal models • Phase II study – early clinical trial to determine safety and efficacy • Target enrollment = 40 patients • Stopped early due to slow enrollment at a single center but a multi-center study is planned JCI 2013 and AJRCCM 2015 24 Drug Repurposing: Anastrazole (Aromatase inhibitor) • Used in the treatment of breast cancer • Prevents conversion of androgens to estrogens • Rationale is to reduce estrogen levels that may contribute to the harmful changes in the pulmonary arteries associated with PAH AJRCCM 2016 25 Drug Repurposing: Anastrazole (Aromatase inhibitor) • Pilot randomized, placebo-controlled study • Primary outcome was change in blood levels of estradiol as well as effect on the right ventricle • 12 PAH patients treated with anastrazole and 6 treated with placebo • Duration was 3 months AJRCCM 2016 26 Drug Repurposing: Anastrazole (Aromatase inhibitor) • Anastrazole reduced blood estrogen levels, increased 6 MWD and did not change measures of right heart function by echo • There were no serious side effects and no differences in side effects between the two groups AJRCCM 2016 27 Drug Repurposing: Spironolactone (Aldactone) • In patients with left heart failure, spironolactone improves blood vessel function, inflammation and survival • Spironolactone treatment is beneficial in PH animal models Trials 2013 28 Drug Repurposing: Spironolactone (Aldactone) Baylor University: Effect of spironolactone on blood levels of collagen/fibrosis markers as well as safety and tolerability Brigham and Women’s Hospital: Effect of ambrisentan (Letaris®) + spironolactone on exercise capacity NIH Clinical Center: Effect of spironolactone on exercise capacity, clinical worsening and inflammation NCT01468571, NCT02253394 and NCT01712620 29 Drug Repurposing: Rituximab • Depletes immune cells that make antibodies • Phase II study in patients with Scleroderma associated PAH • Primary outcome is change in pulmonary vascular resistance • A subset of patients will undergo cardiac MRI to determine effects on the right ventricle NCT01086540 30 AJRCCM 2016 LARIAT: Bardoxolone Methyl for PAH Study Design Abstract Bardoxolone methyl • Nrf2 activator and NF-κB suppressor, targets dysfunctional inflammatory, metabolic, and bioenergetic pathways • US-only Phase II double-blind, randomized, placebo-controlled trial • WHO Group I patients required to be on 1-2 background PAH-specific therapies • Part 1: Dose-ranging (2.5, 5, 10, 20 mg – n=8 per dose group) once daily • Part 2: Open-label extension Primary Study Endpoints • Change in 6MWD from baseline through 16 weeks • Safety and tolerability • Determine the dose range for further clinical trials Oudiz R, et al. Chest. 2015;148(4_MeetingAbstracts):639A. 31 Abstract LARIAT: Bardoxolone Methyl in PAH Primary Endpoint Mean Change in 6MWD Primary Study Endpoint 25 21.4 † 21.6 * Change in 6MWD, m 20 15 10 5 0.2 0 Absolute Change 6MWD Placebo-corrected treatment effect Placebo Phase 2, placebo-controlled dose-ranging study. Combined efficacy analysis. No dose response noted. *P<0.001. †P=0.037 Oudiz R, et al. Chest. 2015;148(4_MeetingAbstracts):639A. 32 Group 4 PH: Balloon Percutaneous Angioplasty (BPA) in CTEPH • Organized thrombi is forced on one side of the vessel enlarging the lumen • BPA causes local dissection of the media with thinning (arrowheads) of the vascular wall leading to the expansion of the luminal diameter over time • Experience using this technique is largely confined to Japan • Experts suggest that this technique may be appropriate for patients with residual PH following PEA • Risk of dissection if PEA follows BPA Kitani, et al. Circ Cardiovasc Interv. 2014;7:857-859. 33 Abstract Efficacy of Balloon Pulmonary Angioplasty in Patients with Inoperable CTEPH CI 25 Before PVR After 6 7.0 3.7 0 Before After BNP (pg/ml) PVR (WU) 8 2 2.7 2.4 350 300 250 200 150 100 50 0 90 80 70 88 92 Before After 60 BNP P<0.01 P<0.01 100 Before 10 4 3.5 3 2.5 2 1.5 1 0.5 0 SaO2 (mmHg) 38 CI (l/min/m2) mPAP (mmHg) 60 50 40 30 20 10 0 SaO2 P<0.01 P<0.01 After 6MWD P<0.01 6MWD (m) mPAP 156 41 Before After 700 600 500 400 300 200 100 0 P<0.01 488 383 Before After N=72 inoperable CTEPH patients undergoing BPA. Mean follow-up period = 21 months. Aoiki T, et al. American Thoracic Society. San Francisco CA. May 13-18, 2016. A1229. 34 Types of Clinical Trials Observational Study • Choose a convenient study population • Collect data over time or review data from prior records • Look for patterns or associations • Generate new hypotheses Interventional Study • Carefully selected participants • Participants and the researchers may be “blinded” • Typically includes a control group to compare to the intervention group 35 What Are The Benefits Of Participating In A Clinical Trial? • Generate knowledge that will help us understand, diagnose and/or treat PH now or in the future • Without clinical research we are unable to improve our understanding of human disease • Early access to new tests or treatments • Frequent contact with health care providers 36 What Are The Risks Of Participating In A Clinical Trial? • Early access to new tests or treatments • Side effects from medications or adverse effects from study procedures • Frequent contact with health care providers • Time investment • The test or treatment may be ineffective or even harmful 37 How Do I Learn More About Current PH Studies? Ask your PH health care team! 38 https://clinicaltrials.gov/ or ask your PH specialist! 39 https://clinicaltrials.gov/ or ask your PH specialist! 40 Questions?? Abubakr Bajwa, MD Mark Rumbak, MD 41