* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download john dalton - sced350akdeniz
Survey
Document related concepts
Transcript
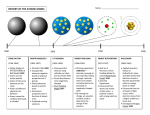
SCED 350.01 SUMMARY OF THE PRESENTATİONS NAME:EZGİ AKDENİZ DUE DATE:20.11.2009 THALES Thales was an ancient Greek philosopher born in Abdera in the north of Greece. His exact contributions are difficult to solve from his advisor Leucippus. Their hypothesis on atoms is remarkably similar to modern science's understanding of atomic structure, and avoided many of the errors of their contemporaries.The theory of Democritus and Leucippus held everything to be composed of atoms, which are physically, but not geometrically, indivisible; that between atoms lies empty space; that atoms are indestructible; have always been, and always will be, in motion; that there are an infinite number of atoms, and kinds of atoms, which differ in shape, size, and temperature. JOHN DALTON John Dalton was an English chemist, meteorologist and physicist. He works on the development of modern atomic model and his reseach into color blindness. The best five points of Dalton's atomic model; The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative atomic weights. All atoms of a given element are identical. Atoms of one element can combine with atoms of other elements to form chemical compounds; a given compound always has the same relative numbers of types of atoms. Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process; a chemical reaction simply changes the way atoms are grouped together. Elements are made of tiny particles called atoms. Also, Dalton said that; Stuff can be broken into elements ( the things listed on the preiodic table). Elements are atoms with different masses Compound are a combinations of elements (like water, salt or pizza). J. JOHN THOMSON Joseph John Thomson was a British physicist and Nobel laureate, credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer. He was awarded the 1906 Nobel Prize in Physics for the discovery of the electron and his work on the conduction of electricity in gases.Thomson played with cathode rays. These are just beams of electrons (but cathode ray sounds cooler). By having the beam interact with electric and magnetic fields, Thomson wa able to determine the mass to charge ratio for an electron. So, from that he knew that the electron came from the atom, it had a negative charge a small mass. Thomson took the idea of the atom and tried to incorporate the evidence for the electron. In this model, the electrons are the small things and the rest of the stuff is some positive matter . Here is the model that he proposed. Here is a model that he proposed. ERNEST RUTHERFORD Ernest Rutherford was a New Zealand chemist and physicist who became as the father of nuclear physicist. He discovered that atoms have their positive charge concentrated in a very small nucleus. He was awarded the Nobel Prize in Chemistry in 1908. He published his atomic theory describing the atom has having a central positive nuclesus surrounded by negative orbiting electrons. This model suggested that most of the mass of the atom was contained in the small nucleus, and that the rest of the atom was mostly empty space. Rutherford came to this conclusion following the results of his famous gold foil experiment. This experiment involved the firing of radioactive particles through minutely thin metal foils (notably gold) and detecting them using screens coated with zinc sulfide NIEL BOHR Niel Bohr was a Danish physicist who made fundamental contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in Copenhagen. He was part of a team of physicists working on the Manhattan Project. In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose energy and spiral into the nucleus. To remedy the stability problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Here are three different light sources. Here is the essence of the bohr model; MARIE CRUIE She was born in 1867. She and his wife began investigating the phenomenon of radioactivity recently discovered in uranium ore. Although the phenomenon was discovered by Henri Becquerel, the term radioactivity was coined by Marie. After chemical extraction of uranium from the ore, Marie noted the residual material to be more "active" than the pure uranium. She concluded that the ore contained, in addition to uranium, new elements that were also radioactive. This led to their discoveries of the elements of polonium and radium, but it took four more years of processing tons of ore under oppressive conditions to isolate enough of each element to determine its chemical properties.For their work on radioactivity, the Curies were awarded the 1903 Nobel Prize in physics. DMITRI MENDELEV Mendelev was a Russion chemist and inventor.He is credited as being the creator of the first version of the periodic table of elements.Using the table, he predicted the properties of elements yet to be discovered. The patterns Mendeleev documented are called a symmetry and whenever there is symmetry in nature, it means that there's a simpler way of describing things. With the elements, the patterns in the masses were a clue that all the elements are made up of smaller particles and their differing masses are simply determined by how many of those particles they have inside them.Mendeleev's idea of the periodic table is a perfect example of a good scientific theory. First, it simplified things enormously, and second it allowed Mendeleev to correctly predict new elements. Element 101, mendelevium, is named in his honour. ERWİN RUDOLF JOSEF ALEXANDAR SCHRÖDİNGER Schrödinger was born Austrian theoretical physicist who achieved fame for his contributions to quantum mechanics, especially the Schrödinger equation, for which he received the Nobel Prize in 1933. In 1935, after extensive correspondence with personal friend Albert Einstein, he proposed the Schrödinger's cat thought experiment.Schrodinger's view of the atom can be seen as "layers within layers" in terms of the electron shells. While not an accurate physical picture of what is happening with the electrons, it does allow us to visually grasp an otherwise difficult concept.Each electron shell is made up of a number of subshells. The number of subshells in a shell is the same as the shell number.So the first shell has only one subshell. The second shell is made of two subshells, the third by three and so on.Erwin Schrodinger added the final piece to the puzzle of electron arrangement around the nuclei of atoms. He suggested that electrons behave in a wave-like manner rather than just as particles and that their exact location within an orbit could not be precisely calculated. This uncertainty principle is complex and fascinating, but to understand the behaviour of atoms in reactions we do not need to go into the detail of it. WERNER HEİSENBERG Heisenberg was born was a German theoretical physicst who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory. In addition, he also made important contributions to nuclear physics, quantum field theory, and particle physics. Classical physics had always assumed that precise location and velocity of objects was always possible. Heisenberg, however discovered that this was not necessarily the case at the atomic level. In particular, he stated that the act of observation interfered with the location and velocity of small particles such as electrons. This is the case because observation requires light and light has momentum. When light bounces off an electron momentum exchange can occur between light and the electron which means the electrons location and velocity have been altered by the act of measurement. This scenerio has important implications to what we can measure at the atomic level. These are probability distributions for the different energy levels in an atom