* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Herpes Zoster Vaccination
Neonatal infection wikipedia , lookup
Poliomyelitis eradication wikipedia , lookup
Kawasaki disease wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Behçet's disease wikipedia , lookup
Infection control wikipedia , lookup
Hepatitis B wikipedia , lookup
Germ theory of disease wikipedia , lookup
Herd immunity wikipedia , lookup
Meningococcal disease wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Whooping cough wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Immunocontraception wikipedia , lookup
Globalization and disease wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Vaccination policy wikipedia , lookup
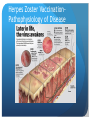
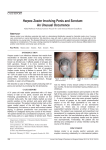
Herpes Zoster Vaccination Anupama Raghuram, MD Assistant Professor Department of Internal Medicine Division of Infectious Diseases August 7th, 2013 Herpes Zoster Vaccination Principles of disease: Virus- Herpes varicella zoster Varicella Zoster (VZV) : 1ry infection Chicken-pox Herpes Zoster( HZV): Reactivation of infection Shingles Herpes Zoster VaccinationPathophysiology of Disease Herpes Zoster VaccinationPathophysiology of Disease Herpes Zoster VaccinationPathophysiology of Disease Transmission: - Direct contact with lesions - Aerosolization of virus from skin lesions - Pt not infectious prior to 1st lesions appearing/ after reepithelization of lesions - Person w/o h/o 1ry VZV exposed to HZV risk of 1ry VZV Herpes Zoster Vaccination Clinical manifestations: Herpes Zoster VaccinationPathophysiology of Disease Precautions with active lesions: - Keep rash covered - Hand washing - Avoid contact with: a) pregnant women w/o h/o VZV b) premature low birth weight infants c) immunocompromised individuals - Isolation: a) localized zoster- not needed b) disseminated zoster- strict isolation ( airborne) Herpes Zoster VaccinationPathophysiology of Disease Complications: - Post- herpetic neuralgia (PHN)- 10-15% of pts - Superimposed bacterial skin infections - Ocular complications- acute retinal necrosis - Neurologic complications: motor neuropathy meningitis, encephalitis myelitis GBS stroke syndrome - Herpes zoster oticus- Ramsay Hunt Herpes Zoster VaccinationPathophysiology of Disease Diagnosis: - Clinical - Viral cx - Direct immunofluorescence - PCR Herpes Zoster Vaccination Why vaccinate? - 1 million cases/ year in the US - $ 1 billion/ year spent on treating HZV - Pain and suffering (rx not always effective/ available, 72 h window) - Prevention of complications Herpes Zoster Vaccination Data on vaccination: A) Shingles Prevention Study (SPS) - 38,546 adults - incidence of HZV by 51% - incidence of PHN by 66.5% B) Zostavax Efficacy and Safety Trial (ZEST) - 22,439 adults 50-59 yo - 2/1,000 person-yrs vs 6.6/ 1,000 person- yrs in placebo group (30 vs 99) - vaccine efficacy for prevention 70% Herpes Zoster Vaccination Mechanism of action: boosts VZV- specific immunity Indications: - ACIP- > 60 yo - FDA- approved for > 50 yo ● Vaccine: - Live attenuated - One time SQ injection- 0.65 ml; - Keep frozen and use within 30 min - 14 x larger dose compared to VZV - Verification of prior zoster history or serology not necessary - Adverse effects: HA, local injection site reaction Herpes Zoster Vaccination Special situations: A) Episode of prior zoster: - if not acute- OK to give vaccine - if recent acute HZV, benefit of vaccine unclear B) Planned immunosuppression: - give HZV vaccination at least 14 d prior Herpes Zoster Vaccination Special situations: C) Pt due for VZV, given HZV vax mistakenly: - count as 1 dose of VZV, proceed with schedule - AVOID- the 2 vaccines are NOT interchangeable D) Pt due for HZV, given VZV instead - dose much lower in VZV, do not count as a valid dose - if mistake picked up during the visit, give HZV then - mistake picked up later, give HZV after 28 days Herpes Zoster Vaccination ● Contraindications: - Pregnant women - Anaphylactic reaction to gelatin or neomycin - 1ry or acquired immunodeficiency (BM malignancy, AIDS, steroids) - Avoid co-administration with PPSV23 ( Pneumovax): immunogenicity of zoster vaccine References 1. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Oxman et al. Shingles Prevention Study Group.N Engl J Med. 2005;352(22):227 2. http://www.cdc.gov/shingles/about/transmission.html 3. http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi.pdf 4. Recommended adult immunization schedule: United States, 2010. Advisory Committee on Immunization Practices. Ann Intern Med. 2010;152(1):36. 5. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Schmader KE et al. JClin Infect Dis. 2012 Apr;54(7):922-8. 6. Airborne transmission of nosocomial varicella from localized zoster. Josephson A, Gombert ME. J Infect Dis. 1988;158(1):238 7. Rapid contamination of the environments with varicella-zoster virus DNA from a patient with herpes zoster. Yoshikawa T et al. J Med Virol. 2001;63(1):64. 8. Transmission of varicella zoster virus from individuals with herpes zoster or varicella in school and day care settings.Viner K et al. J Infect Dis. 2012 May;205(9):1336-41. Epub 2012 Mar 28.