* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biosafety - Portal UniMAP
Sexually transmitted infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Hepatitis B wikipedia , lookup
African trypanosomiasis wikipedia , lookup
History of biological warfare wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Biological warfare wikipedia , lookup
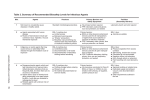
ERT 322 SAFETY AND LOSS PREVENTION BIOSAFETY AND BIOHAZARD SAFETY Overview • • • • • Biohazard Biosafety rules & regulations Risk Assessment Decontamination Protection Biohazard • A biological hazard or biohazard is an organism, or substance derived from an organism, that poses a threat to (primarily) human health. • This can include medical waste or samples of a microorganism, virus or toxin (from a biological source) that can impact human health. Four Levels of Biosafety • BSL 1: Material not known to consistently cause disease in healthy adults. • BSL 2: Associated with human disease. Hazard is from percutaneous injury, ingestion, or mucous membrane exposure. • BSL 3: Indigenous or exotic agents with potential for aerosol transmission; disease may have serious or lethal consequences. • BSL 4: Dangerous/exotic agents which pose a high risk of life-threatening disease, aerosol-transmitted lab infections or related agents with unknown risk of transmission. Biosafety Level 1 • agents not known to cause disease in healthy adult humans • minimal potential hazard to laboratory personnel and the environment. • For microbes example, Escherichia coli, Bacillus subtilis, Naegleria gruberi. • Work is generally conducted on open bench tops using standard microbiological practices • all materials used for cell and/or bacteria cultures are decontaminated via autoclave. Biosafety Level 2 • moderate potential hazard to personnel and the environment. • For microbes example, Hepatitis B virus, Influenza A, Measles virus, dengue fever, Salmonella. • certain procedures in which infectious aerosols or splashes may be created are conducted in biological safety cabinets or other physical containment equipment. Biosafety Level 3 • infectious agents which may cause serious or potentially lethal disease as a result of exposure by the inhalation route. • Exposure potential to pathogens spread by aerosol. • Infection serious, possibly lethal • For microbes example, Coxiella burnetii, Monkeypox virus, SARS, antrax. • All procedures involving the manipulation of infectious materials are conducted within biological safety cabinets Biosafety Level 4 • Suitable for work with dangerous and exotic agents that pose a high individual risk of aerosol- transmitted laboratory infections and life-threatening disease. • Exposure potential to pathogens spread by aerosol or with unknown risk of transmission. • Infection possibly lethal. • For microbes example, Ebola Zaire, Marburg virus • When dealing with biological hazards at this level the use of a Hazmat suit and a selfcontained oxygen supply is mandatory. Rules & Regulations • OSHA Bloodborne Pathogens http://www.osha.gov/SLTC/bloodbornepathogens/index.html • CDC Select Agents http://www.cdc.gov/od/ohs/lrsat.htm • NIH Guidelines for Research Involving Recombinant DNA Molecules http://www4.od.nih.gov/oba/rac/guidelines/guidelines.html • DOT/CDC Shipping http://www.cdc.gov/od/ohs/biosfty/shipregs.htm • CDC Import Permits http://www.cdc.gov/od/ohs/biosfty/imprtper.htm • USDA/APHIS Permits http://www.aphis.usda.gov/vs/ncie/ Import Permits • CDC: - a permit is required to import etiologic agents of human disease and any materials, including live animals or insects, that may contain them. - Unsterilized specimens of human and animal tissues (such as blood, body discharges, fluids, excretions or similar material) containing an infectious or etiologic agent require a permit in order to be imported. Import Permits • APHIS: a USDA veterinary permit is needed for materials derived from animals or exposed to animal-source materials. Risk Assessment Risk of Activity – same agent can have different containment levels at different stages of protocol: – Procedures that produce aerosols have higher risk – Procedures using needles or other sharps have higher risk – Handling blood, serum or tissue samples may have lower risk – Purified cultures or cell concentrates may have higher risk – Larger volumes (10 L) have higher risk Decontamination Decontamination -a process or treatment that renders an instrument or environmental surface safe to handle. - Decontamination procedure can range from sterilization to simple cleaning with soap and water. - Sterilization, disinfection and antisepsis are all forms of decontamination. Sterilization is the use of a physical or chemical procedure to destroy all microbial life, including highly resistant bacterial endospores. Antisepsis is the application of a liquid antimicrobial chemical to skin or living tissue to inhibit or destroy microorganisms. It includes swabbing an injection site on a person or animal and hand washing with germicidal solutions. Disinfection eliminates virtually all pathogenic non-sporeforming microorganisms but not necessarily all microbial forms on inanimate objects (e.g., work surfaces and equipment). Effectiveness is influenced by the kinds and numbers of organisms, the amount of organic matter, the object to be disinfected and chemical exposure time, temperature and concentration. Types of disinfectants – alcohol, aldehydes, oxidizing agent & phenolics. Methods of Decontamination • There are four main categories of physical and chemical means of decontamination: (1) Heat; (2) Liquid disinfection; (3) Vapors and gases; and (4) Radiation. 1. Heat Wet Heat Wet heat is the most dependable method of sterilization. Autoclaving (saturated steam under pressure of approximately 15 psi (1.02atm) to achieve a chamber temperature of at least 250°F (121˚C) for a prescribed time) is the most convenient method of rapidly achieving destruction of all forms of microbial life. The material to be sterilized must come in contact with steam and heat. Dry Heat Dry heat is less efficient than wet heat and requires longer times and/or higher temperatures to achieve sterilization. It is suitable for the destruction of viable organisms on impermeable non-organic surfaces such as glass, but it is not reliable in the presence of shallow layers of organic or inorganic materials which may act as insulation. 2. Liquid Disinfection The most practical use of liquid disinfectants is for surface decontamination and, as a decontaminate for liquid wastes prior to final disposal in the sanitary sewer. Liquid disinfectants are available under a wide variety of trade names. In general, these can be classified as: halogens, acids, alkalis, heavy metal salts, quaternary ammonium compounds, phenolic compounds, aldehydes, ketones, alcohols and amines. 3. Vapors & Gases Vapors and gases are primarily used to decontaminate : biological safety cabinets and associated systems; bulky or stationary equipment not suited to liquid disinfectants; instruments or optics which might be damaged by other decontamination methods; and rooms, buildings and associated airhandling systems. Agents included in this category are: - glutaraldehyde and formaldehyde vapor, - ethylene oxide gas, - peracetic acid - hydrogen peroxide vapor. When used in closed systems and under controlled conditions of temperature and humidity, excellent disinfection can be obtained. 4. Radiation • Although ionizing radiation will destroy microorganisms, it is not a practical tool for laboratory use. • Nonionizing radiation in the form of ultraviolet radiation (UV) is used for inactivating viruses, bacteria and fungi. • It will destroy airborne microorganisms and inactivate micro-organisms on exposed surfaces or in the presence of products of unstable composition that cannot be treated by conventional means. • UV can cause burns to the eyes and skin of people exposed for even a short period of time. Therefore, proper shielding should be maintained when it is in use. Protection: Primary Containment • Lab practices – standard lab practice, - limited access, - biohazard warning sign, - sharps/needle precautions, - SOPs for decontamination, waste, medicals. • Safety equipment – biosafety cabinets (BSC), sharps containers, sealed rotors. • Personal protective equipment (PPE) – lab coat, gloves, goggles. Aerosol Precautions • Use BSC for all procedures that may generate aerosols. • Use centrifuges with biosafety covers. • Do not use a syringe for mixing infectious fluids. • Cultures, tissues, specimens of body fluids, etc., are placed in a container with a cover that prevents leakage during collection, handling, processing, storage, transport or shipping. Human Blood, Tissue and Fluid Occupational Exposure to Bloodborne Pathogens Occupational Safety & Health Administration (OSHA) 29 CFR 1910.1030 • Use BSL 2 work practices and procedures. • Everyone needs to be offered the Hepatitis B vaccine. • Develop specific exposure plan SOPs. • Specific training is required. • Review needle/syringe use and replace with “safe” devices. • Exposure incidents must be followed up. Waste Disposal • “Red bag” or “Regulated Medical Waste”: – All mammalian cells or anything that came in contact with mammalian cells – All BSL 2 material or anything that came in contact with BSL 2 material – All needles/syringes regardless of use Environmental Safety & Health Overview: Who has to protect Exposure Response Plan • To protect employees from exposure to the pathogenic microorganism. • describe ways to eliminate or minimize the danger of exposure to human blood or other potentially infectious materials. • Must be specific to the department • Update yearly • Accessible to workers • • • •