* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download proforma for registration of subjects for pg dissertation
Survey
Document related concepts
Discovery and development of proton pump inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Compounding wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Transcript
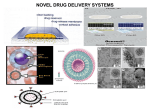
NIOSOMES OF NATIGLINIDE SYNOPSIS FOR M.PHARM DISSERTATION SUBMITTED TO RAJIV GANDHI UNIVERSITY OF HEALTH SCIENCES BANGALORE, KARNATAKA. BY NAGARAJ.P Ι M.PHARM DEPARTMENT OF PHARMACEUTICS, M.E.S. COLLEGE OF PHARMACY, BANGALORE-562110 KARNATAKA. (2010-2011) ANNEXURE-II PROFORMA FOR REGISTRATION OF SUBJECTS FOR P.G. DISSERTATION 1. NAME OF THE CANDIDATE AND NAGARAJU POTLABATHINI ADDRESS c\o: M.E.S COLLEGE OF PHARMACY, (IN BLOCK LETTERS) BANGALORE-560054. 2. NAME OF THE INSTITUTION M.E.S COLLEGE OF PHARMACY, BANGALORE-560054. 3. COURSE OF STUDY AND SUBJECT MASTER OF PHARMACY IN PHARMACEUTICS 4. DATE OF THE ADMISSION 5. TITLE OF THE TOPIC: NIOSOMES OF NATIGLINIDE BRIEF RESUME OF THE INTENDED WORK 6.1 NEED FOR THE STUDY In the part two and half decades several advancements have been made in the field of pharmaceutics. This has resulted in the development of new techniques for drug delivery. Niosomes are one of the novel drugs delivery systems which are microscope lamellar structures formed on admixture of cholesterol and single alkyl chain non- toxic surfactant with subsequent hydration in aqueous media (J.N Khandaree ; Indian drugs, 2001) These are the non toxic surfactant vesicle which can entrap both hydrophilic and lipophillic drugs, either in aqueous layer (or) in vesicular membrane made up of lipid material. ADVANTAGES OF NIOSOMES . Use of noisome in cosmetics was first done by L’oreal as they offered the following advantages. The vesicle suspension being water based offers greater patient compliance over oil based systems Since the structure of the noisome offers place to accommodate hydrophilic, lipophillic as well as amphiphilic drug moieties, they can be used for a variety of drugs. The characteristics such as size, lamellarity etc; of the vesicle can be varied depending on the requirement. The vesicles can act as a depot to release the drug slowly and offer a controlled release. Other advantages of noisome are : They are osmotically active and stable They increase the stability of the entrapped drug Handling and storage of surfactants do not require any special conditions Can increase the oral bioavailability of drugs Can enhance the skin preparations of drugs They can be used for oral, parenteral, as well as topical use The surfactants are biodegradable, biocompatible and non – immunogenic Improve the therapeutic performance of the drug by protecting it from the biological environment and restricting effects of target cells, thereby reducing the clearance of the drug. Nateglinide (INN,trade name starlix) is a drug for the treatment of type 2 diabetes. I t is a phenylalanine derivative, non sulfonylurea which belongs to the class of Meglitinide. PHYSICO CHEMICAL PROPERTIES Molecular structure Chemical name :(R)-2-(4-isopropylcyclohexanecarboxamido)-3-phenylpropanoic acid Molecular formula : C19H27NO3 Molecular weight : 317.423 g/mol Category : Anti-diabetic Description : white colour ,odourless and crystalline powder. Solubility : Freely soluble in methanol, ethanol, and chloroform; soluble in ether; sparingly soluble in acetonitrile and octanol; practically insoluble in water. PHARMACOKINETIC DATA Absorption: Rapidly absorbed following oral administration prior to a meal.Administration with or after a meal results in a decrease in the peak plasma concentration (C max), and a delay in the time to reach the C max (Tmax). However, there is no change in the area under the plasma concentration–time curve (AUC). Distribution: Absolute bioavailability is approximately 73%. Following intravenous administration to healthy volunteers, the steady-state volume of distribution (VolD) was approximately 10 L.Nataglinide has a low affinity for heart and skeletal muscle tissue. Protein binding: Very high (98%); primarily to serum albumin and to a lesser extent to alpha 1–acid glycoprotein. Biotransformation: Metabolism is via hydroxylation followed by glucuronidation. The major metabolites have less antidiabetic activity than nateglinide, but the isoprene minor metabolite has antidiabetic activity comparable to that of nateglinide.Cytochrome P450 isoenzymes CYP2C9 and CYP3A4 have been shown in vitro to be involved in the metabolis of nateglinide. Half-life: Elimination— 1.5 hours. Onset of action: 20 minutes. Time to peak concentration: 1 hour. Duration of action: 4 hours. Elimination: Renal, 83% (75% within 6 hours after dosing). Approximately 16% excreted as parent compound. Fecal, approximately 10%. 6.2 REVIEW OF LITERATURE Deepika Aggarwal and Indu P. Kaur formulated chitosan (REVTMbio1) or Carbopol (REVTMbio2 and 3) coated niosomal timolol maleate (0.25%) by reverse phase evaporation (REV) and compared to timolol solution (TMS; 0.25%) in terms of in vitro release and IOP lowering pharmacodynamic effect. The in vitro release phase of timolol (91% release in 2 h) was extended significantly by its incorporation into niosomes and further by the polymer coating (40–43% release up to 10 h). REVTMbio1 formulation showed a more sustained effect of upto 8 h (vis a vis 6 h for carbopol-coated niosomes). TMS in comparison showed effect for only 2 h though the peak effect was slightly more (14%). Lowering of IOP in the contralateral eye (20–40% as compared to 100% in case of TMS), considerably reduces with REV and REVbio formulations indicating lesser systemic side effects. Rita Muzzalupo et al synthesised of α,ω-trioxyethylene-bis(sodium 2-dodecyloxypropylenesulfonate), an anionic Gemini surfactant, and its ability to form niosomes are elucidated. The compound forms vesicles with and without added cholesterol. The vesicular systems were characterized by size, shape and drug entrapment efficiency. The compounds to be incorporated are β-carotene and ferulic acid, as anti oxidants, acetyl salicylic acid, as FANS, and the antineoplastic 5flurouracil, widely used in dermatological disorders. The results of this study show that α,ωtrioxyethylene-bis(sodium 2-dodecyloxy-propylenesulfonate) can be used for the preparation of niosomes entrapping lypophilic, amphiphilic or hydrophilic substances. These niosomes may be promising candidates as percutaneous carriers for the aforementioned drugs. Biswajit Mukherjee et al designed to develop and compare acyclovir containing nanovesicular liposomes and niosomes based on cholesterol, soya L-α-lecithin and nonionic surfactant, span 20. The effort was made to study in vitro whether acyclovir-loaded nanovesicles could sustain the release of the drug by increasing residence time and thus, acyclovir could reduce its dose-related systemic toxicity. There were good vesicular distributions in both of the niosomes and the liposomes. The obtained vesicles were within 1 μm and about 35% of them were within a size of 100 nm. The percentage of drug loading varied and the niosomal vesicles contained more drug as compared with the liposomes. When the in vitro drug release was compared, it was found that the liposomes released about 90% drug in 150 min whereas the drug release was just 50% from the niosomal vesicles in 200 min. Again, the niosomes showed better stability compared with the liposomes. Thus, niosome could be a better choice for intravenous delivery of acyclovir. Shyamala Bhaskaran and Lakshmi P K prepared salbutamol sulphate niosomes using Span 60 as the surfactant, by employing different techniques namely, thin film hydration, hand shaking, ether injection, lipid layer hydration and trans membrane pH gradient method. The drug encapsulation efficiency varied from 62 % to 87 %. In vitro drug release studies was carried out and formulation exhibited retarded release for 24 h. Transmembrane pH gradient method was found to be most satisfactory which released 78.4 % of drug in 24 h. This formulation was lyophilized and characterized by infrared spectroscopy. Tissue distribution studies in albino rats and bioavailability studies in rabbits were carried out. Waraporn Suwakul et al formulated Propylthiouracil niosomes using various classes of nonionic surfactants. Feasibility of vesicle formation by the sonication method was evaluated. Size and size distribution was measured by laser diffraction. Entrapment and drug release over 24 h were monitored by UV spectrophotometric method at 275 nm. The results revealed that niosomes readily formed from various compositions of nonionic surfactant and cholesterol, with or without a stabilizer. Entrapment of PTU in niosomes depended on bilayer composition. The release of PTU from all niosomal formulations studied was retarded and followed the first-order kinetics. Degree of slow release had a negative correlation with drug entrapment. The release rate also depended on the physical state of the bilayer. The results of this study indicate that PTU niosomes were able to control the release of PTU and might be of value to develop further into topical formulations. Satturwar P M et al prepared Ketoconazole niosomes by thin film hydration technique for topical application using surfactant (Tween 40 or 80), cholesterol and drug in five different ratios (by weight) viz. 1:1:1, 1:5:1:1, 2:1:1, 2.5:1 and 3:1:1. The prepared niosomes were characterized for size, shape, entrapment efficiency and in vitro drug release (by exhaustive dialysis). Niosomes were then formulated in FAPG base and tested for in vitro antifungal activity (cup plate method). The studies demonstrated successful preparation of Ketoconazole niosomes offering considerable advantage over plain drug formulation. Lakshmi P K et al formulated a novel sustained release niosomal 0.25% MTX using a polymer chitosan administered once daily for 12 weeks. It was compared with placebo gel and plain MTX 0.25% gel for the treatment of different types of psoriasis, especially palmoplantar psoriasis. 30 patients were enrolled for study. They were divided randomly in to three groups of 10 patients for each formulation. The patients with 25% or less than 25% psoriatic lesions were included for the study.The results are calculated using PASI scoring. Changes in the disease signs and symptoms indicated that both agents have anti psoriatic activity but not with placebo gel. However lesions treated with Niosomal chitosan-MTX formulation showed marked improvement in comparison to plain MTX and placebo gel inspite of twice a day application. Few patients experienced mild adverse events. No clinically significant changes in blood or other lab parameters wereseen. The findings suggest that the 0.25% niosomal MTX in chitosan gel exhibited beneficial effect in psoriasis and did not exert any systemic toxicity. Ghada Abdelbary and Nashwa El-gendy designed and investigated the feasibility of using non-ionic surfactant vesicles (niosomes) as carriers for the ophthalmic controlled delivery of a water soluble local antibiotic; gentamicin sulphate. Niosomal formulations were prepared using various surfactants (Tween 60, Tween 80 or Brij 35), in the presence of cholesterol and a negative charge inducer dicetyl phosphate (DCP) in different molar ratios and by employing a thin film hydration technique. The ability of these vesicles to entrap the studied drug was evaluated by determining the entrapment efficiency %EE after centrifugation and separation of the formed vesicles. Photomicroscopy and transmission electron microscopy as well as particle size analysis were used to study the formation, morphology and size of the drug loaded niosomes. Results showed a substantial change in the release rate and an alteration in the %EE of gentamicin sulphate from niosomal formulations upon varying type of surfactant, cholesterol content and presence or absence of DCP. In-vitro drug release results confirmed that niosomal formulations have exhibited a high retention of gentamicin sulphate inside the vesicles such that their in vitro release was slower compared to the drug solution. A preparation with 1:1:0.1 molar ratio of Tween 60, cholesterol and DCP gave the most advantageous entrapment (92.02% ± 1.43) and release results (Q8h = 66.29% ± 1.33) as compared to other compositions. Ocular irritancy test performed on albino rabbits, showed no sign of irritation for all tested niosomal formulations. Hunter C A et al designed Niosomal and liposomal drug formulations which were equiactive and both increased drug efficacy by an order of magnitude compared with that of free drug. Niosomes containing 30 mol % cholesterol were prepared from three different non-ionic surfactants and no significant difference in activity was detected among the different drug-loaded niosomes. Both negatively charged and neutral vesicles were found to be equally effective. However, vesicle cholesterol content had a slight influence on the antiparasitic activity of the drugloaded niosomes. Empty vesicles produced a dose-dependent parasite suppression for all vesicles studied. Studies of antimony distribution in the mouse using neutron activation analysis showed high liver levels after i.v. administration of the carrier forms of the drug. 6.3 OBJECTIVES OF THE STUDY: The aim of the present work is, Formulation of Niosome tablets of natiglinide using suitable optimization technique and to optimize the drug release profile. Characterization of the prepared niosomes of natiglinide tablets. To evaluate the optimized formulations for various in vitro parameters like in vitro dissolution, studies, niosomes strength studies. Predicting the drug release mechanism. To subject the optimized formulations for stability studies as per ICH guidelines. Statistical analysis of all the results. MATERIALS AND METHOD 7.1 SOURCE OF DATA 1. Library: M.E.S College of Pharmacy. 2. e-library: M.E.S College of Pharmacy. 3. The data will be collected from official books such as IP,BP,USP. 4. Internet source. 5. RGUHS Library, Bangalore (J-Gate) 7.2 MATERIALS: 1. Natiglinide 2. Span60 3. methanol, diethylether 4. sodium hydroxide 5.potassiumdihydrogenphosphate. Hand shaking method . In this method surfactant and cholesterol mixture was dissolved in a common solvent (diethyl ether).By taking the mixture in a round bottomed flask, the solvent was removed at room temperature (20ºc) under reduced pressure in a rotary evaporator. The dried surfactant film was hydrated with the aqueous phase containing the drug at 50-60ºc with gentle agitation. The obtained niosomal dispension was subjected to dialysis, or centrifugation, gel chromatography (Hirai G.S et al 1989). Azmin et al modified this method for preparing methotrexate entrapped niosomes. The ingredients were dissolved in chloroform-methanol mixture (1:1, 2ml v/v) and the solvent was evaporated at room temperature using a rotary evaporator. The thin layer of solid mixture deposited on the wall of round bottomed flask was hydrated with the aqueous phase at 7 0C for 15 minutes. This suspension was sonicated for 3×30 sec to form unilamellar vesicles. This method produces multilamellar niosomes with largest diameter. 7.3 METHODS OF DATA COLLECTION: 1. Literature survey using internet and scientific Journals. 2. Data will be collected from experimental studies which includes: The compatibility studies of drug and polymers using thermal analysis such as FTIR, and DSC techniques. Preformulation studies. Formulation development and evaluation. Optimization of the niosomes of natiglinide tablets using suitable optimization technique. In vitro strength studies, in vitro studies. Spectrophotometric method for the estimation of drug and for analysis of in-vitro dissolution samples and for the determination of drug loading efficiency. Predicting the drug release mechanism by curve fit method. Statistical analysis of all the results. Stability studies as per ICH guidelines. 7.4 Does the study require any investigation or intervention to be conducted on patients or other human or animals? -NO- 7.5 Has ethical clearance been obtained from your institute? -NOT APPLICABLE- REFERENCES: . Agarwal D et al, Devolopment of topical niosomal preparation of acetazolamide; preparation and evaluation . J. Pharm. Pharmacol. 2004, 56(12): 1509. 2. Aranya Manosroi* , Pensak Jantrawut , Narinthorn Khositsuntiwong , Worapaka Manosroi and Jiradej Manosroi , Novel Elastic Nanovesicles for Cosmeceutical and Pharmaceutical Applications, Chiang Mai J. Sci. 2009; 36(2) : 168-178 3. Arunothayanun P et al., The effect of processing variables on the physical charecteristics of nonionic surfactant vesicles (niosomes) formed by hexadecyl diglycerol ether.Int.J. Pharm, 2000, 201:7. 4. Azmin MN et al , The effect of nonionic surfactantvesicle (noisome)entrapment on the absorption and distribution of methotrexate in mice.J.Pharm .Pharmacol,1985,37:237. 5. Bal Subramanian A et al, Formulation and invivo evaluation of noisome encapsulated daunorubicin hydrochloride. Drugdev and Ind. Pharm. 2002, 28:1181. 6. Ballie AJ et al, The preparation and properties of niosomes –nonionic surfactant vesicles .J .Pharm .Pharmacol ,1985, 37:863. 13. 7. Biswajit Mukherjee, Balaram Patra, Buddhadev Layek, and Arup Mukherjee. Int J Nanomedicine. 2007 June; 2(2): 213–225. 8. Carter K. C, Dolan T. F, Alexander I, Bailie A.J. and Mc. Colgan C, J. Pharm. Pharmacol. 1989, 41,87 9. chandra prakash K.S at al., 1990,61,R1-R3 10. Chauhar S and Lawprence M. J. J. Pharm . Pharmacol. Sppl 1989, 41, 6p. 11. Christine Dufes, Frederic Gaillard, Ijeoma F. Uchegbu, Andreas G. Schätzlein, Jean-Christophe Olivier and Jean-Marc Muller, Glucose-targeted niosomes deliver vasoactive intestinal peptide (VIP) to the brain, International Journal of Pharmaceutics Volume 285, Issues 1-2, 5 November 2004, Pages 77-85. 12. Deepika Aggarwal; Alka Garg; Indu P. Kaur, Development of a topical niosomal preparation of acetazolamide: preparation and evaluation, Journal of Pharmacy and Pharmacology, Volume 56, Number 12, December 2004 , pp. 1509-1517(9). 13. d' souza at al.,J.pharm. Pharmacol,1997,49(feb);145-149. 14. Dreamer.D, Banghan. A. B., I.Bdi., 1976, 443, 629-634. 15. s.erdogan at al., S.T.P. pharma.,sciences 6(1) 87-93,2996. 16. elsie oomen at al., J.pharm.pharmacol.commun 1999, 8,281-285 17. Elise Oommen, B Dinesh Shenoy, N.Udupa, Ravindra Kamanth and P.Umadevi.J .A., J. Control. Rel .1992, 21, 145. 18. Ghada Abdelbary and Nashwa El-gendy, Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery, Received: 3 March 2008 Accepted: 10 April 2008 Published online: 18 June 2008 19. H hopli and minis nair Ind.j.pharm.sci., august,1996, 163-166 20. Hafland H .e.J . A. Verhoef J. C, Bucj towg, chowdary B. Z. Ponee M. Junginger H. E. J, J. Pharm. Pharmacol, 1992, 44, 287. 21. Hirari G.S, Yashiki.T , Mima, Int. J. Pharm . 1989, 91, 65. 22. Hunter CA et al, Safety aspect of nonionic surfactant vesicles- a toxicity study related to the physicochemical charecteristics of nonionic surfactants. J Pharm.pharmacol. 1988, 40:161. 23. J.N.Khandarre,Jiwan Das Bobade Hemant and U.R.Ramesh; Indian drugs, 2001,38(4),197-202. 24. Khandarre, J.N.Madhavi, S.N; Eastren Pharmacist, 1995, 175-176 25. Kippenberg d, Rosenquist K, Odberg L., Fendler J. H. Am< Chem. Soc. 193, 105. 26. Kirby C, Clarke, J. Gregorioadis. G., Biochem J.1980, 186, 591. 27. Kreuter J. Colloidal Drug delivery system .66; Niosome. dekker series p73, Dekker publication. 28/ K. Ruckmani ; B. Jayakar ; S. K. Ghosal Drug Development and Industrial Pharmacy, Volume 26, Issue 2 January 2000 , pages 217 - 222 . 29. Manosroi A et al, Sorbitan monostearate / polysorbate20 organogels containing niosomes; a delivery vehicle of antigen. European J. Pharm. Sci, 2003, 8: 177. 30. Mayer L.D. Hope M. J, Cllius P.R., Chem Phys. Lipids,1985, 40, 333. 31. Namedeo Alok, Mishra, P.R.Khopade. A.J. and N.K. Jain. Indian drugs, 1993, 30(6),378-380. 32. Nasseri B and Florence At. Microtubules formed by capillary extrusion and fusion of surfactant vesicles. Int..J of Pharm. 2003, 266:91. 33. Nasseri B and Florence AT. Some properties of extruded nonionic surfactant microtubes.I SIGNATURE OF THE CANDIDATE 10 REMARKS OF THE GUIDE 11 NAME AND DESIGNATION OF Recommended to carry out the dissertation 11.1 GUIDE Mr. V.B.MAHESH BABU M.Pharm Asst. Professor Dept. of Pharmaceutics M.E.S College of Pharmacy, Bangalore-560054. 11.2 SIGNATURE NOT APPLICABLE 11.3 CO-GUIDE 11.4 SIGNATURE 11.5 HEAD OF THE DEPT. DR.CHANDRAPRAKASH Ph.D, Professor and Head Dept. of Pharmaceutics M.E.S College of Pharmacy, Bangalore-560054. 11.6 SIGNATURE 12 REMARKS OF THE PRINCIPAL 12.1 SIGNATURE M.Pharm