* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HIV
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
HIV vaccine wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
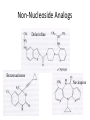
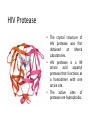
Antivirals for HIV Yasir Waheed, PhD Some HIV Facts • HIV – the Human Immunodeficiency Virus is the retrovirus that causes AIDS • HIV belongs to the retrovirus subfamily lentivirus. • HIV attaches to cells with CD4 receptors (T4 cells and macrophages). HIV Life • • • • • • • • • • 1 Cycle Step 1: Attachment of virus at the CD4 receptor and chemokine co-receptors CXCR4 or CCR5 Step 2: viral fusion and uncoating Steps 3-5: Reverse transcriptase makes a single DNA copy of the viral RNA and then makes another to form a double stranded viral DNA Step 6: migration to nucleus Steps 7-8: Integration of the viral DNA into cellular DNA by the enzyme integrase Steps 9-11: Transcription and RNA processing Steps 12-13: Protein synthesis Step 14: protease cleaves polypeptides into functional HIV proteins and the virion assembles Step 15: virion budding Step 16: Virion maturation Anti- HIV Drug Targets2 Three types of drugs are currently in clinical use: 1. nucleoside and nucleotide reverse transcriptase (RT) inhibitors 2. non-nucleoside reverse transcriptase inhibitors 3. protease inhibitors (PIs) Nucleoside and Nucleotide Analogs • Nucleoside analogs (NRTI) act as chain terminators or inhibitors at the substrate binding site of RT • NRTI’s must be phosphorylated (three steps) to their 5’-triphosphate form to become active inhibitors. • Nucleotide analogs (NtRTI) already contain a phosphate group and only go through 2 steps to become active. • The 5’-triphosphate of the NRTI’s compete with the 2’-deoxynucleoside’s 5’-triphosphate for binding to reverse transcriptase leading to viral DNA chain termination3. Nucleoside Analogs • There are currently 7 FDAapproved NRTI’s and one nucleotide analog. • The first anti-HIV drug approved was the NRTI known as AZT or Zidovudine (1987). • AZT was discovered as a treatment of AIDS during a screening process for the identification of effective AIDS treatments. • Antiviral selectivity due to higher affinity for HIV RT than human DNA polymerases. Non-Nucleoside Analogs • Non-nucleoside analog reverse transcriptase inhibitors (NNRTI’s) inhibit viral DNA replication by binding at the allosteric non-bonding site of RT, causing a conformational change of the active site. • NNRTI’s do not require bioactivation by kinases. • Three NNRTI’s are currently approved for clinical use in combination therapy: nevirapine, delavirdine, and efavirenz. Non-Nucleoside Analogs Delavirdine Benzoxazinone Nevirapine Protease Inhibitors • During the reproduction cycle of HIV a specific protease is needed to process the polyproteins into mature HIV components. • If protease is missing non-infectious HIV is produced. • HIV protease inhibitors are specific to HIV protease because it differs significantly from human protease. • The 6 PI’s currently approved for clinical use were all designed by using structure-based drug design methods. HIV Protease • The crystal structure of HIV protease was first obtained at Merck Laboratories. • HIV protease is a 99 amino acid aspartyl protease that functions as a homodimer with one active site. • The active sites of protease are hydrophobic. Protease Inhibitors • ABT-378 or lopinavir was approved in 2000 for use in combination with ritonavir (a PI) (Kaletra) • Ritonavir strongly inhibits the metabolism of ABT378. Some Alternative Therapies • Virus adsorption inhibitors – interfere with virus binding to cell surface by shielding the positively charged sites on the gp-120 glycoprotein – Polyanionic compounds • Viral coreceptor antagonists – compete for binding at the CXCR4 (X4) and CCR5 (R5) coreceptors – bicyclams and ligands Virus Adsorption Inhibitors • Cosalane was originally developed as an anticancer agent by researchers at Purdue University and the U.S. National Cancer Institute. • Cosalane was developed from a chemical known as ATA (aurintricarboxylic acid), which has long been known to have anti-HIV activity. • The result was cosalane. • Cosalane binds to the HIV gp-120 protein. Viral Coreceptor Antagonists • Bicyclams are a type of viral coreceptor antagonist. • They are very specific and potent X4 coreceptor antagonists. • Bicyclams belong to a class of macrocyclic polyamines consisting of two cyclam units linked by an aliphatic bridge • Bicyclams with an aromatic linker apparently had higher antiviral activity. • One such compound is AMD3100. Combination Therapy • Combination therapy often called HAART is standard care for people with HIV. • Monotherapy created virus resistance to the individual drug. Some combination therapies increase the time it takes for the virus to become resistant. • Combinations of a PI or NNRTI with one or two NRTI’s is often recommended. • Combination therapy may reduce individual drug toxicity by lowering the dosage of each drug Drug Toxicity and Side Effects • All available antiretroviral drugs are toxic. • Side effects of nucleoside analogs are lactic acidosis and severe hepatomegaly with steatosis (enlarged fatty liver). • Other side effects of anti-HIV drugs include pancreatitis, myopathy, anemia, peripheral neuropathy, nausea, and diarrhea. Reducing Drug Toxicity • The use of combination therapy: – Combining agents with favorable synergistic properties allows a decrease in dose or dosing frequency – Ritonavir alone cause gastrointestinal side effects but when used in combination with other PI’s it can be administered at a lower dose. 10 Million Patients on Antiretroviral Therapy 2013 Global AIDS Response Progress Reporting (WHO/UNICEF/UNAIDS) 18 Principles of HIV Drug Resistance • Not all drug failure is due to resistance • Partial HIV suppression promotes resistance • Resistance may fade but not disappear when a drug is stopped 19 Principles of Resistance (2) • Some mutations allow certain viruses to resist the effects of one or more antiretroviral drugs. • Each infected person has a mixture of viruses, some of which are resistant to some medications. • The drug resistant virus usually grows faster and better than the drug susceptible virus. • The drug resistant virus replaces the drug susceptible virus in the patient. 20 Resistance Testing • Two types: – Genotyping • Less expensive • Can usually be completed in 1-2 weeks – Phenotyping • More expensive • Generally takes 2-3 weeks to complete 21 Suspect Resistance in the Setting of Treatment Failure • Due to HIV’s high transcription error rate and high level of replication, mutant HIV variants constantly generated. • These variants often contain mutations that confer variable levels of resistance to antiretroviral agents. • Poor adherence or suboptimal regimens can lead to resistance and ‘viral breakthrough’. 22 How Does Resistance Develop? • Results from changes (mutations) in the genetic information in the virus. • These changes occur whenever HIV is replicating. • Every possible mutation occurs tens of thousands of times each day. 23 Resistance Mutations • For some drugs (NNRTIs and 3TC), a single mutation causes high-level resistance. – Resistance to these drugs occurs very quickly • For other drugs (most NRTIs and PIs), many mutations must occur before high-level resistance is observed. – Resistance to these drugs occurs more slowly 24 Cross-Resistance • Resistance to one drug can cause resistance to others of the same class – NNRTI: complete cross-class resistance – NRTI: partial cross-class resistance – PI: partial cross-class resistance • Partly overcome by ritonavir boosting 25 Minimize Emergence of Viral Resistance • Never prescribe ARVs in the absence of adherence counseling and support • Never prescribe monotherapy or dual therapy • Ensure optimal serum drug concentrations – Avoid drug interactions – Diagnose and manage malabsorption • If ARV medications are to be discontinued, stop all drugs at the same time – Possible exception: NNRTI-based regimen 26 2003 vs. 2005 WHO Guidelines 27 THANKS