* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anti-HIV Drugs
Survey
Document related concepts
Discovery and development of direct Xa inhibitors wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
HIV vaccine wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Transcript
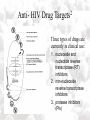
Anti-HIV Drugs Cathy Molina November 11, 2004 Some HIV Facts • HIV – the Human Immunodeficiency Virus is the retrovirus that causes AIDS • HIV belongs to the retrovirus subfamily lentivirus. • HIV attaches to cells with CD4 receptors (T4 cells and macrophages). HIV Life Cycle1 • • • • • • • • • • Step 1: Attachment of virus at the CD4 receptor and chemokine co-receptors CXCR4 or CCR5 Step 2: viral fusion and uncoating Steps 3-5: Reverse transcriptase makes a single DNA copy of the viral RNA and then makes another to form a double stranded viral DNA Step 6: migration to nucleus Steps 7-8: Integration of the viral DNA into cellular DNA by the enzyme integrase Steps 9-11: Transcription and RNA processing Steps 12-13: Protein synthesis Step 14: protease cleaves polypeptides into functional HIV proteins and the virion assembles Step 15: virion budding Step 16: Virion maturation Anti- HIV Drug 2 Targets Three types of drugs are currently in clinical use: 1. nucleoside and nucleotide reverse transcriptase (RT) inhibitors 2. non-nucleoside reverse transcriptase inhibitors 3. protease inhibitors (PIs) Nucleoside and Nucleotide Analogs • Nucleoside analogs (NRTI) act as chain terminators or inhibitors at the substrate binding site of RT • NRTI’s must be phosphorylated (three steps) to their 5’-triphosphate form to become active inhibitors. • Nucleotide analogs (NtRTI) already contain a phosphate group and only go through 2 steps to become active. • The 5’-triphosphate of the NRTI’s compete with the 2’-deoxynucleoside’s 5’-triphosphate for binding to reverse transcriptase leading to viral DNA chain termination3. Nucleoside Analogs • There are currently 7 FDAapproved NRTI’s and one nucleotide analog. • The first anti-HIV drug approved was the NRTI known as AZT or Zidovudine (1987). • AZT was discovered as a treatment of AIDS during a screening process for the identification of effective AIDS treatments4. • Antiviral selectivity due to higher affinity for HIV RT than human DNA polymerases. Non-Nucleoside Analogs • Non-nucleoside analog reverse transcriptase inhibitors (NNRTI’s) inhibit viral DNA replication by binding at the allosteric non-bonding site of RT, causing a conformational change of the active site. • NNRTI’s do not require bioactivation by kinases. • Three NNRTI’s are currently approved for clinical use in combination therapy: nevirapine, delavirdine, and efavirenz 5 Non-Nucleoside Analogs Delavirdine Benzoxazinone Nevirapine Protease Inhibitors • During the reproduction cycle of HIV a specific protease is needed to process GAG and POL polyproteins into mature HIV components. • If protease is missing noninfectious HIV is produced. • HIV protease inhibitors are specific to HIV protease because it differs significantly from human protease. • The 6 PI’s currently approved for clinical use were all designed by using structure-based drug design methods4. HIV 6 Protease • The crystal structure of HIV protease was first obtained at Merck Laboratories. • HIV protease is a 99 amino acid aspartyl protease that functions as a homodimer with one active site. • The active sites of protease are hydrophobic. Protease 7 Inhibitors • HIV PI’s target the peptide linkages in the gag and gag-pol polyproteins which must be cleaved by protease. • All approved PI’s contain a hydroxyethylene bond instead of a normal peptide bond. • The hydroxyethylene bond makes PI’s non-scissile substrate analogs for HIV protease Protease 7 Inhibitors • ABT-378 or lopinavir was approved in 2000 for use in combination with ritonavir (a PI) (Kaletra) • Ritonavir strongly inhibits the metabolism of ABT378 Some Alternative Therapies • Virus adsorption inhibitors – interfere with virus binding to cell surface by shielding the positively charged sites on the gp-120 glycoprotein – Polyanionic compounds • Viral coreceptor antagonists – compete for binding at the CXCR4 (X4) and CCR5 (R5) coreceptors – bicyclams and ligands Virus Adsorption Inhibitors • • • • • Cosalane was originally developed as an anti-cancer agent by researchers at Purdue University and the U.S. National Cancer Institute8. Cosalane was developed from a chemical known as ATA (aurintricarboxylic acid), which has long been known to have anti-HIV activity8. ATA is a mixture of different polymers. Chemists took one of the low molecular weight components of ATA, and attached it to a steroid molecule in order to target the substance more effectively to the surface of viruses and of cells. The result was cosalane. Cosalane binds to the HIV gp-120 protein. Viral Coreceptor Antagonists • Bicyclams are a type of viral coreceptor antagonist. • They are very specific and potent X4 coreceptor antagonists. • Bicyclams belong to a class of macrocyclic polyamines consisting of two cyclam units linked by an aliphatic bridge • Bicyclams with an aromatic linker apparently had higher antiviral activity10. • One such compound is AMD3100. Combination Therapy • Combination therapy often called HAART is standard care for people with HIV. • Monotherapy created virus resistance to the individual drug. Some combination therapies increase the time it takes for the virus to become resistant. • Combinations of a PI or NNRTI with one or two NRTI’s is often recommended. • Combination therapy may reduce individual drug toxicity by lowering the dosage of each drug Combination Therapy • The combination of drugs chosen is based on the history of each individual patient and synergistic drug interactions. • Some drugs compete with each other for binding sites or enzymes. – Example: zidovudine and stavudine • both nucleoside analogs compete for the same kinase. Stavudine is not phosphorylated because zidovudine is preferred5. Combination Therapy and Drug Resistance • Some drug combinations can restore sensitivity of the virus to drugs it was previously resistant to. – Example: lamivudine and zidovudine • The HIV M184V mutation is resistant to lamivudine but restores sensitivity to zidovudine resistant virus mutants5. Drug Toxicity and Side Effects • All available antiretroviral drugs are toxic. • Side effects of nucleoside analogs are lactic acidosis and severe hepatomegaly with steatosis (enlarged fatty liver)11. • Other side effects of anti-HIV drugs include pancreatitis, myopathy, anemia, peripheral neuropathy, nausea, and diarrhea. Reducing Drug Toxicity • The use of combination therapy: – Combining agents with favorable synergistic properties allows a decrease in dose or dosing frequency – Ritonavir alone cause gastrointestinal side effects but when used in combination with other PI’s it can be administered at a lower dose. Conclusions • An effective anti-HIV therapy is still needed. • Several possible targets are being studied and tested. • The area of anti-HIV drugs has more room for growth and the future for the discovery of new effective drugs is promising. References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. NIAID HIV Life Cycle. http://www.niaid.nih.gov/daids/dtpdp/virpage1.htm (accessed Oct 2004). De Clerq, E. New anti-HIV agents and targets. Med. Res. Rev. 2002, 22(6), 531-565. El Kouni, M. H. Trends in the design of nucleoside analogues as anti-HIV drugs. Current Pharmaceutical Design. 2002, 8(8), 581-593. Block, J. H.; Beale, J. M. Antiviral Agents, Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 11th ed; Lippincott Williams & Wilkins: Maryland, 2004; pgs 379, 943. De Clerq, E.; Vandamme, A-M. Combination Therapy of AIDS. Birkhauser Verlag: Germany, 2004. Brik, A.; Wong, C-H. HIV-1 protease: mechanism and drug discovery. Organic & Biomolecular Chemistry. 2003, 1(1), 5-14. De Clerq, E. New Developments in Anti-HIV Chemotherapy. Current Medicinal Chemistry. 2001, 8, 1543-1572. cosalane website – look up Ruell, J. A.; De Clercq, E.; Pannecouque, C. Synthesis and Anti-HIV Activity of Cosalane Analogues with Substituted Benzoic Acid Rings Attached to the Pharmacophore through Methylene and Amide Linkers. J. Org. Chem. 1999, 64, 5858-5866. Labrosse, B.; Brelot, A.; Heveker, N.; Sol, N. Determinants for Sensitivity of Human Immunodeficiency Virus Coreceptor CXCR4 to the Bicyclam AMD3100. J. Virol. 1998, 6381–6388. Simple FactSheet from the AIDS Treatment Data Network. http://www.atdn.org/simple/abac.html (accssed Nov 2004).