* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Evolutionary history of life wikipedia , lookup
Soil salinity control wikipedia , lookup
River ecosystem wikipedia , lookup
Surface runoff wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Water pollution wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Plant nutrition wikipedia , lookup
Soil microbiology wikipedia , lookup
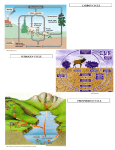
Ch. 4 - Ecology, Ecosystems and Food Webs Ecology - five levels of matter in nature (overhead) Organisms > Populations > Communities > Ecosystems > Ecosphere Parts of the Earth Core - inner and outer (iron) Mantle - includes the asthenosphere Lithosphere - upper mantle and crust Atmosphere thermosphere mesosphere stratosphere - ozone layer troposphere - lower atmosphere (climate) Hydrosphere - water Biosphere - ecosphere What sustains life on earth? one-way flow of energy cycling of matter and nutrients gravity Open and closed systems: Open: flow of energy and matter in and out of the system Closed: only flow of energy into and out of the system. No flow of matter Sun: Nuclear fusion Natural Greenhouse Effect: greenhouse gases are water, carbon dioxide, methane, nitrous oxide and ozone. Ecosystems: land: Biomes (determined largely by the climate) - see Video: BIOMES water: Aquatic Life Zones Boundaries of an ecosystem - ECOTONES Components of an Ecosystem: Abiotic - nonliving (water, air, nutrients, solar energy) Biotic - living -( plants and animals) Range of Tolerance (Tolerance limits) Limiting Factor principle Major Living components of an ecosystem Producers (autotrophs) photosynthesis chemosynthesis Consumers Herbivores - plant eaters PRIMARY - feed directly on producers Carnivores secondary - feed only on primary consumers (herbivores) tertiary - feed only on other carnivores Omnivores - eat plants and animals 1 Other consumers: scavengers: Detritivores Decomposers Respiration Aerobic Anaerobic Food Webs and Energy Flow in Ecosystems Food Chain: high quality energy (sunlight) is converted to nutrients by photosynthesis. This energy is passed on to consumers and eventually decomposers. Low quality heat is emitted into the environment Trophic Level: feeding level producers 1st trophic level primary consumers 2nd trophic level secondary consumers 3rd trophic level etc. Food Web is an interconnected food chains (complex) 1. grazing food webs 2. detrital food webs Biomass Ecological Efficiency - the percentage of energy transferred from one trophic level to another. a 10% efficiency means that 90% of the energy is lost. Pyramids of Energy Flow - illustrate energy loss in a food chain help explain how the Earth can support more people if people would eat more grains, vegetables, etc., and not eat consumers of those grains (steer, deer, etc.) Top level carnivores (eagles, hawks, tigers, sharks) are few in number and are the first to suffer when the ecosystems are disrupted. - making them especially vulnerable to extinction. Storage of biomass at various trophic levels can be represented by a pyramid of biomass. Pyramid of Numbers Gross Primary Productivity (GPP) - the rate at which an ecosystem's producers convert solar energy into chemical energy as biomass 2 Net Primary Productivity (NPP) - biomass that is left after producers use some for their own use. How do Ecologists learn about Ecosystems? Field Research Lab Research Systems Analysis Systems Measurement Data Analysis Systems Modeling Systems Simulation Systems Optimization – Ecosystem Services and Sustainability Why is Biodiversity such an important ecosystem service? The two Basic Principles of Ecosystem Sustainability 1. 2. Soils Soil - mixture of eroded rock, minerals, organic matter, water, air, living organisms (decomposers) very slow to renew Horizons O - litter A - topsoil B - subsoil Profiles Soil Texture Clay - silt -sand - gravel Relative amounts determine texture of the soil porosity volume of the pores permeability - determined by the average size of the pores in the soil See soil triangle in text. Soil texture, porosity and permeability determine a soil's 1) water-holding capacity, 2) aeration or oxygen content, and 3) workability. (ease of cultivation) Soil acidity (pH) - influences the uptake of nutrients 3 Nutrient Cycling Rainfall - can erode soil, dissolve soil nutrients Wind - can blow away topsoil and deposits nutrients elsewhere Nutrients lost from one ecosystem must enter another ecosystem Nutrient Cycles and What are Nutrient (biogeochemical) Cycles? a process by which nutrients are recycled between living organisms and nonliving environment. The three general types of nutrient cycles: Hydrologic Cycle Atmospheric Cycle Sedimentary Cycle The Water Cycle Role of Water - terrestrial: major factor that determines types of organisms that can live there: rain forests and deserts. Aquatic ecosystems: water flow influences temperature, salinity, and availability of nutrients. How is water cycled in the Ecosphere? evaporation transpiration condensation precipitation infiltration percolation runoff Humidity - the amount of water in the air - depends on the temperature of the air. Absolute humidity (g/kg) Relative humidity (%) Precipitation - requires the presence of condensation nuclei (volcanic ash, smoke, sea salts, any particulate matter) Dew Point - the temperature at which condensation will occur Aquifer Water Table 4 Circulation rate - underground water (300-4600 yrs), lakes (13 years), streams (13 days), atmosphere (9 days), ocean (37,000 yrs) and glaciers (16,000 yrs). Many of the natural processes act to purify the water (a natural distillation/filtration process). How people affect the water cycle: 1. we withdraw large quantities of fresh water from lakes, rivers, groundwater, ... 2. we clear vegetation from land for agriculture and other uses (increasing runoff and reducing infiltration) 3. we modify water quality by adding nutrients (phosphates) and other pollutants The Carbon Cycle Carbon - the currency of energy exchange the flow of energy follows the flow of carbon (temp. is proportional to the conc. of CO2) See pp.78-79 for conceptual model of the carbon cycle. Average residence time: atmosphere (3 yrs), soils (25-30 yrs), oceans (1500 yrs). How people affect the carbon cycle: 1. forest and brush removal - less vegetation to remove CO2 through photosynthesis 2. burning fossil fuels The Nitrogen Cycle Role of nitrogen: used by organisms to make vital organic compounds - amino acids, proteins, DNA and RNA. Usually in short supply and limits the rte of primary production. Nitrogen (N2) cannot be absorbed and used directly by plants or animals. most complex of the nutrient cycles. Nitrogen fixation - bacteria convert N2 into NH3 [N2 + 3 H2 ==> 2 NH3]; cyanobacteria in soil and water and by the Rhizobium bacteria living in small nodules on the roots of legumes (soybeans, alfalfa, and clover). - - Nitrification - NH3 is converted to NO2 (toxic to plants) and then to NO3 (readily used by plants). + Assimilation - roots absorb NH3, NH4 and nitrate to make the nitrogen containing compounds. 5 Ammonification - specialized decomposed bacteria convert the nitrogen rich organic compounds (waste, etc.) into simpler nitrogen containing inorganic + compounds (NH3 and NH4 ). + Denitrification - bacteria convert NH3 and NH4 back into nitrite and nitrate and then into N2 and nitrous oxide (N2O).==> which is released into the atmosphere and the process starts all over again. How people affect the Nitrogen Cycle: We react nitrogen with hydrogen to make ammonia (the Haber Process)revolutionizing agriculture. 1. Emit NO when we burn fuel. the NO produces HNO3 (acid rain); create O3 and photochemical smog. 2. Emit heat-trapping nitrous oxide (N2O) into the atmosphere through the action of anaerobic bacteria on livestock waste 3. remove nitrogen from the earth's crust when we mine nitrogen containing mineral deposits for fertilizers. 4. remove nitrogen from topsoil when we burn grasslands and clear forests. 5. add excess nitrogen compounds to aquatic systems in agricultural runoff, discharge of municipal sewage and deposition of nitrogen compounds from the atmosphere. 6. add excess nitrogen compounds to terrestrial ecosystems through atmospheric deposition involving HNO3 and NO2. The Phosphorus Cycle 3- 2- essential nutrient for plants and animals (as PO4 and HPO4 ) How is Phosphorus Cycled in the Ecosphere? Water, crust and in living organisms. In Sedimentary Cycle: phosphate deposits on land and shallow ocean deposits to living organisms and then back to the land and ocean. Phosphorus is found as phosphate salts in rock and is released by weathering of the rock, is dissolved in soil water and taken up by plant roots. Phosphorus and its compounds are not gaseous so P is not circulated in the atmosphere. Phosphorus cycle is very slow. Most soils are low in phosphorus, so P is the limiting factor for plant growth (so fertilizer is needed). How people affect the Phosphorus Cycle (3): 6 1. we mine large quantities of phosphate rock for fertilizers and detergents 2. cutting down the rain forests reduces available Phosphate. In rain forests, all phosphate is found in the plants and animals, not the soil. P is washed away when forests are cut and burned. 3. we add excessive phosphate to aquatic ecosystems in runoff of animal wastes from livestock feedlots, runoff of commercial fertilizers The Sulfur Cycle Sulfur is a component of most proteins and some vitamins. S enters atmosphere in several natural sources. H2S from active volcanoes and breakdown of organic matter in swamps, bogs, tidal flats. SO2 (from volcanoes) 2- SO4 salts from sea spray. How people affect the Sulfur Cycle: 1. burning sulfur-containing coal and oil to produce electricity 2. refining petroleum 3. by using smelting to convert sulfur compounds of metallic minerals into free metals (Cu, Pb, Zn), 4. other industrial processes. Rock Cycle - How rocks change over time Slowest of all Earth's cycles Three types of rocks: Igneous Sedimentary Metamorphic 7