3quarksdaily: More Is Different
... and the atom bursts, a genuine classical electron flies out. The electron, as it leaves the atom, crystallizes out of Schrödinger's mist like a genie emerging from his bottle." In countless other physical scenarios, we find new properties surfacing on macroscopic scales - more is different, as Phili ...
... and the atom bursts, a genuine classical electron flies out. The electron, as it leaves the atom, crystallizes out of Schrödinger's mist like a genie emerging from his bottle." In countless other physical scenarios, we find new properties surfacing on macroscopic scales - more is different, as Phili ...
7302 (Analytical Dynamics)
... Analytical dynamics develops Newtonian mechanics to the stage where powerful mathematical techniques can be used to determine the behaviour of many physical systems. The mathematical framework also plays a role in the formulation of modern quantum and relativity theories. Topics studied are the kine ...
... Analytical dynamics develops Newtonian mechanics to the stage where powerful mathematical techniques can be used to determine the behaviour of many physical systems. The mathematical framework also plays a role in the formulation of modern quantum and relativity theories. Topics studied are the kine ...
Chern-Simons theory and the fractional quantum Hall effect
... makes the bridge between the classical and quantum theory once the canonical quantization approach is considered, where roughly speaking the observables are promoted to operators acting on quantum states in a Hilbert space, and the Poisson structure is mapped into a commutation relations between the ...
... makes the bridge between the classical and quantum theory once the canonical quantization approach is considered, where roughly speaking the observables are promoted to operators acting on quantum states in a Hilbert space, and the Poisson structure is mapped into a commutation relations between the ...
5.62 Physical Chemistry II
... properties in terms of microscopic atomic and molecular properties. These ...
... properties in terms of microscopic atomic and molecular properties. These ...
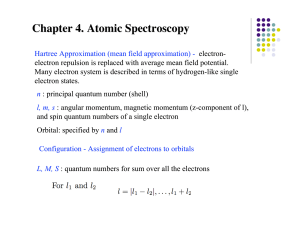
3.1 The correspondence principle
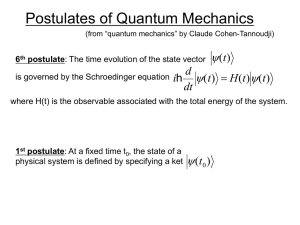
... The calculated energy Eigenvalues En and Eigenvectors fn define how the quantum mechanical system evolves in time: Let for t = 0 the system be in a state X ψ(0, ~ri ) = cn fn ...
... The calculated energy Eigenvalues En and Eigenvectors fn define how the quantum mechanical system evolves in time: Let for t = 0 the system be in a state X ψ(0, ~ri ) = cn fn ...
Quantum mechanics is the theory that we use to describe the
... spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is that spin is quantised. It can only have discrete values. For example, protons, neutrons and electrons are all “spin half” particles, that is, they have spin values that are one half H ...
... spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is that spin is quantised. It can only have discrete values. For example, protons, neutrons and electrons are all “spin half” particles, that is, they have spin values that are one half H ...
Quantum Mechanics Lecture 1 Dr. Mauro Ferreira
... • Consider the following experiment: “classical” particles are allowed through a narrow gap. The blue curve displays how they are spatially distributed ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
... • Consider the following experiment: “classical” particles are allowed through a narrow gap. The blue curve displays how they are spatially distributed ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
Answer
... values from 0 to n − 1. In this case n = 2, so the allowed values of the angular momentum quantum number are 0 and 1. Each allowed value of the angular momentum quantum number labels a subshell. Within a given subshell (label l) there are 2l + 1 allowed energy states (orbitals) each labeled by a dif ...
... values from 0 to n − 1. In this case n = 2, so the allowed values of the angular momentum quantum number are 0 and 1. Each allowed value of the angular momentum quantum number labels a subshell. Within a given subshell (label l) there are 2l + 1 allowed energy states (orbitals) each labeled by a dif ...
Quantum Mechanics
... 2. Consider a particle of mass M constrained to move on a circle of radius a in the x, y-plane. a. Write down the Schrödinger equation in terms of the usual cylindrical-polar angle φ. b. Determine the complete set of states, the corresponding energy spectrum and orthonormalize the stationary states ...
... 2. Consider a particle of mass M constrained to move on a circle of radius a in the x, y-plane. a. Write down the Schrödinger equation in terms of the usual cylindrical-polar angle φ. b. Determine the complete set of states, the corresponding energy spectrum and orthonormalize the stationary states ...
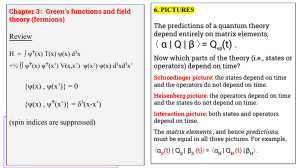
α | Q | β 〉= Q (t) . 〈 Review
... The initial and final states , i.e., as t ⟶ −∞ and +∞, are free particles, i.e., eigenstates of H0. The state experiences the interactions H1 during the time -1/ε ≲ t ≲ +1/ε . ...
... The initial and final states , i.e., as t ⟶ −∞ and +∞, are free particles, i.e., eigenstates of H0. The state experiences the interactions H1 during the time -1/ε ≲ t ≲ +1/ε . ...
The Quantum Mechanical Behavior of Light and Matter
... smallest value of r obtained from n=1 (ground state of atomic hydrogen) ...
... smallest value of r obtained from n=1 (ground state of atomic hydrogen) ...
Toffoli gate
... It can be shown that if two observables are measured simultaneously, the uncertainty in their joint values must always obey the inequality (Heisenberg ...
... It can be shown that if two observables are measured simultaneously, the uncertainty in their joint values must always obey the inequality (Heisenberg ...