Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... according to some random radioactive decay). This leads to the cat paradox, as in reality we have never seen a macroscopic superposition. What is the justification for the superposition state: It is the only way we know to explain some experimental observations! ...
... according to some random radioactive decay). This leads to the cat paradox, as in reality we have never seen a macroscopic superposition. What is the justification for the superposition state: It is the only way we know to explain some experimental observations! ...
ACS Seminar on Internet computing Internet Security Issues
... algorithms will not compromise the security provided by systems using these technologies. Quantum cryptography is also safe with respect to future advances in code breaking and computing, including developments in the new field of quantum computing, which will severely reduce or possibly obliterate ...
... algorithms will not compromise the security provided by systems using these technologies. Quantum cryptography is also safe with respect to future advances in code breaking and computing, including developments in the new field of quantum computing, which will severely reduce or possibly obliterate ...
PHYS 305 - Modern Physics (Spring 2016) Department of Physics
... - Demonstrations. The following general education goals and objective will be addressed by this course: • Effective communication through speaking and writing. • Critical thinking and problem solving. • Incorporate computational intelligence and knowledge for solving problems. METHODS OF EVALUATION ...
... - Demonstrations. The following general education goals and objective will be addressed by this course: • Effective communication through speaking and writing. • Critical thinking and problem solving. • Incorporate computational intelligence and knowledge for solving problems. METHODS OF EVALUATION ...
Instructions for Preparing Abstracts for MS+S2004
... three-junction flux qubit [2] is one of such candidates. On the basis of fundamental qubit operations [3,4], the cavity QED like experiments are possible on a superconductor chip by replacing an atom with a flux qubit, and a high-Q cavity with a superconducting LC-circuit. By measuring qubit state j ...
... three-junction flux qubit [2] is one of such candidates. On the basis of fundamental qubit operations [3,4], the cavity QED like experiments are possible on a superconductor chip by replacing an atom with a flux qubit, and a high-Q cavity with a superconducting LC-circuit. By measuring qubit state j ...
PDF
... Hamiltonian algebroids are generalizations of the Lie algebras of canonical transformations, but cannot be considered just a special case of Lie algebroids. They are instead a special case of a quantum algebroid. Definition 0.1. Let X and Y be two vector fields on a smooth manifold M , represented h ...
... Hamiltonian algebroids are generalizations of the Lie algebras of canonical transformations, but cannot be considered just a special case of Lie algebroids. They are instead a special case of a quantum algebroid. Definition 0.1. Let X and Y be two vector fields on a smooth manifold M , represented h ...
OBJECTIVE WORKSHEET Quantum Theory 1. How did
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
... 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orbitals? 7. What is the maximum number of electrons allowed in when n=4? 8. What is "neon ...
A spectral theoretic approach to quantum
... The results of this work show the ubiquity of quantum integrability, in strong contrast with classical integrability. In fact, and due to various arguments (existence of invariant cylinders in quantum phase space or of infinite conservation laws, linearity and functions of eigenprojectors . . . ), ...
... The results of this work show the ubiquity of quantum integrability, in strong contrast with classical integrability. In fact, and due to various arguments (existence of invariant cylinders in quantum phase space or of infinite conservation laws, linearity and functions of eigenprojectors . . . ), ...
Quiz 4
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
Theory of quantum state control with solid-state qubits Research supervisor
... The potential to exploit quantum-mechanics in technology, from sensors to computers, is vast. Essential for these developments, however, is the ability to take a quantum system with a few discrete states, such as an exciton in a quantum dot or impurity state in a crystal, and control its wavefunctio ...
... The potential to exploit quantum-mechanics in technology, from sensors to computers, is vast. Essential for these developments, however, is the ability to take a quantum system with a few discrete states, such as an exciton in a quantum dot or impurity state in a crystal, and control its wavefunctio ...
Group representation theory and quantum physics
... quantum information. Thus, as group theory becomes trendy again via, for example, the hidden subgroup problem in quantum computing [1], I thought it might be of interest to first revisit the “old” use of group theory in quantum mechanics. Because this is a mathematics seminar, I will spend more time ...
... quantum information. Thus, as group theory becomes trendy again via, for example, the hidden subgroup problem in quantum computing [1], I thought it might be of interest to first revisit the “old” use of group theory in quantum mechanics. Because this is a mathematics seminar, I will spend more time ...
Sec 4-1 Chapter 4 Notes
... In 1926, Erwin Schrodinger developed an equation that treated e- as waves. Together, the Heisenberg uncertainty principle and the Schrodinger equation laid the foundation for the Quantum theory. Heisenberg uncertainty principle gives us a probability of where to find the e-. The e- does not travel ...
... In 1926, Erwin Schrodinger developed an equation that treated e- as waves. Together, the Heisenberg uncertainty principle and the Schrodinger equation laid the foundation for the Quantum theory. Heisenberg uncertainty principle gives us a probability of where to find the e-. The e- does not travel ...
S
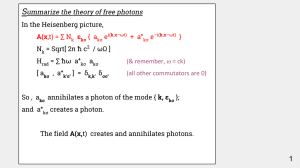
... No, because the expectation values of the quantum fields are a Maxwellian wave. If the number of photons is large, the quantum effects are negligible. ...
... No, because the expectation values of the quantum fields are a Maxwellian wave. If the number of photons is large, the quantum effects are negligible. ...
Path integral in quantum mechanics
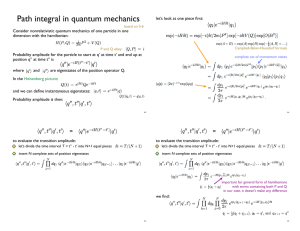
... let’s divide the time interval T = t’’ - t’ into N+1 equal pieces ...
... let’s divide the time interval T = t’’ - t’ into N+1 equal pieces ...
Quantum Theory of Atoms and Molecules
... Electronic structure of atoms. Schrödinger equation for 1-electron atoms and form of solutions. Energy levels, orbitals and their representation, quantum numbers and their interpretation, spin-orbit coupling. Many electron atoms, orbitals and the orbital approximation, energy levels, Pauli exclusion ...
... Electronic structure of atoms. Schrödinger equation for 1-electron atoms and form of solutions. Energy levels, orbitals and their representation, quantum numbers and their interpretation, spin-orbit coupling. Many electron atoms, orbitals and the orbital approximation, energy levels, Pauli exclusion ...
Lectuer 15
... - It takes on the 2 Ɩ + 1 values m = 0, ±1, ±2, ……, ± Ɩ. - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – f ...
... - It takes on the 2 Ɩ + 1 values m = 0, ±1, ±2, ……, ± Ɩ. - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – f ...