Nutritional Pattern Among Orgnaisms
... microbes for synthesis of cellular materials • Protein synthesis nitrogen and sulfur • DNA or RNA synthesis nitrogen, Many bacteria derive nitrogen by decomposing protein phosphorus • ATP synthesis nitrogen and phosphorus • Some bacteria ammonium ions in organic material • nitrogen from nitrates • N ...
... microbes for synthesis of cellular materials • Protein synthesis nitrogen and sulfur • DNA or RNA synthesis nitrogen, Many bacteria derive nitrogen by decomposing protein phosphorus • ATP synthesis nitrogen and phosphorus • Some bacteria ammonium ions in organic material • nitrogen from nitrates • N ...
Writing formulas and naming ionic bonds
... Because it forms a negative ion, it is called an ____. anion ...
... Because it forms a negative ion, it is called an ____. anion ...
Topic 16 Some non-metals and their compounds notes
... catalyst of vanadium(V) oxide (V2O5) at a temperature of 450oC and a pressure of 2 atmospheres. This reaction, in which sulfur trioxide is formed, is exothermic. It is also a redox reaction, because sulfur is oxidized from an oxidation state of +4 to an oxidation state of +6. 2SO2(g) + O2(g) ...
... catalyst of vanadium(V) oxide (V2O5) at a temperature of 450oC and a pressure of 2 atmospheres. This reaction, in which sulfur trioxide is formed, is exothermic. It is also a redox reaction, because sulfur is oxidized from an oxidation state of +4 to an oxidation state of +6. 2SO2(g) + O2(g) ...
Uses of Sulfuric Acid
... Waste products Most of the “waste” heat is recovered and used to heat water, in this way much of the energy can be reused. Because of this many sulfuric acid plants are co-located with other industrial processes. Great care needs to be taken with the waste gases that are formed. There will be small ...
... Waste products Most of the “waste” heat is recovered and used to heat water, in this way much of the energy can be reused. Because of this many sulfuric acid plants are co-located with other industrial processes. Great care needs to be taken with the waste gases that are formed. There will be small ...
The Mineral That Helps Fight Fatigue
... MSM is also highly concentrated in aloe vera, so you can use natural aloe vera products to increase your intake of MSM in its natural form. Another alternative is to take MSM as a dietary supplement. In a previous interview, superfood expert David Wolfe recommended taking about 2,500 mg per day to s ...
... MSM is also highly concentrated in aloe vera, so you can use natural aloe vera products to increase your intake of MSM in its natural form. Another alternative is to take MSM as a dietary supplement. In a previous interview, superfood expert David Wolfe recommended taking about 2,500 mg per day to s ...
Group 16 Elements
... The name comes from the greek ‘oxy genes’ meaning ‘acid forming’. Oxygen is a diatomic element naturally found as a colorless, odorless gas. It is also highly flammable. It makes up 21% of the Earth’s atmosphere, 49% by mass of the Earth’s crust, and ⅔ of the human body. The oxygen content in the at ...
... The name comes from the greek ‘oxy genes’ meaning ‘acid forming’. Oxygen is a diatomic element naturally found as a colorless, odorless gas. It is also highly flammable. It makes up 21% of the Earth’s atmosphere, 49% by mass of the Earth’s crust, and ⅔ of the human body. The oxygen content in the at ...
abstract
... GCA; Osburn. 2013; Dawson et al. 2015. Geobiology], along with product sulfide that is depleted in the heavier isotopes of sulfur relative to reactant sulfate (2εSO4-H2S ~ 0 to 70‰). In recent studies it has been suggested that the magnitude of hydrogen isotopic fractionation may relate to central e ...
... GCA; Osburn. 2013; Dawson et al. 2015. Geobiology], along with product sulfide that is depleted in the heavier isotopes of sulfur relative to reactant sulfate (2εSO4-H2S ~ 0 to 70‰). In recent studies it has been suggested that the magnitude of hydrogen isotopic fractionation may relate to central e ...
multiple choice study questions for this week`s material
... a. it is only a problem in New England and Scandinavia b. it can damage plants and water resources c. it is caused mainly by the release of oxides of sulfur and nitrogen d. can fall to ground in dry or wet forms ____ 20. Another name for acid rain: a. wet deposition b. acid fog c. dry deposition d. ...
... a. it is only a problem in New England and Scandinavia b. it can damage plants and water resources c. it is caused mainly by the release of oxides of sulfur and nitrogen d. can fall to ground in dry or wet forms ____ 20. Another name for acid rain: a. wet deposition b. acid fog c. dry deposition d. ...
1. dia
... • occurs at spring when the melted acidic snow flows suddenly into the rivers of catchments area. • If natural water is in contact with limestone, dolomite, the pH does not change → buffer effect. The living organisms are killed by the increased CO2 content • In case of week buffer effect (small Ca- ...
... • occurs at spring when the melted acidic snow flows suddenly into the rivers of catchments area. • If natural water is in contact with limestone, dolomite, the pH does not change → buffer effect. The living organisms are killed by the increased CO2 content • In case of week buffer effect (small Ca- ...
*Coal often contains sulfur as an impurity that is released as
... Btus per metric ton, determine the number of kg of coal needed to produce 1 million Btus of heat. ...
... Btus per metric ton, determine the number of kg of coal needed to produce 1 million Btus of heat. ...
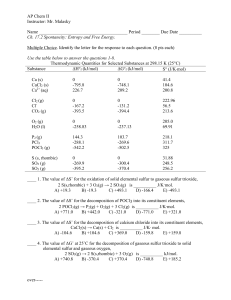
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Sulfur for Ruminants - Westway Feed Products
... Most basic foodstuffs, including forages, contain sulfur as a component of the S-containing amino acids. Sulfur fertilization will increase the S level of plants; forages are ‘luxury accumulators’ of sulfur, meaning that, when available, they will continue to take up additional S beyond the level th ...
... Most basic foodstuffs, including forages, contain sulfur as a component of the S-containing amino acids. Sulfur fertilization will increase the S level of plants; forages are ‘luxury accumulators’ of sulfur, meaning that, when available, they will continue to take up additional S beyond the level th ...
Allergies to Sulfur Compounds?
... The confusion between Sulfates, Sulfites and Sulfa drugs Consumers who are allergic to sulfa drugs or have had reactions to sulfites in foods are often concerned about potential reactions when using dietary supplements containing sulfates or sulfur (i.e., glucosamine sulfate, chondroitin sulfate, MS ...
... The confusion between Sulfates, Sulfites and Sulfa drugs Consumers who are allergic to sulfa drugs or have had reactions to sulfites in foods are often concerned about potential reactions when using dietary supplements containing sulfates or sulfur (i.e., glucosamine sulfate, chondroitin sulfate, MS ...
Upon completion of Chapter 7, you should be able to
... A sample has been found to consist of 54% calcium, 43.2 percent oxygen, and 2.70 percent hydrogen. What is its empirical formula? ...
... A sample has been found to consist of 54% calcium, 43.2 percent oxygen, and 2.70 percent hydrogen. What is its empirical formula? ...
File
... Haber-Bosch Process: A technique for making ammonia from hydrogen and nitrogen, according to the first equation. To get the reactants, nitrogen gas is liquefied form air and hydrogen gas is obtained chemically from methane (natural gas). First natural gas is treated to remove sulfur-containing compo ...
... Haber-Bosch Process: A technique for making ammonia from hydrogen and nitrogen, according to the first equation. To get the reactants, nitrogen gas is liquefied form air and hydrogen gas is obtained chemically from methane (natural gas). First natural gas is treated to remove sulfur-containing compo ...
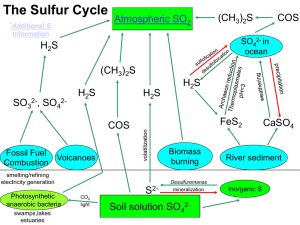
The Carbon Cycle : The different forms and compounds in which
... Haber-Bosch Process: A technique for making ammonia from hydrogen and nitrogen, according to the first equation. To get the reactants, nitrogen gas is liquefied form air and hydrogen gas is obtained chemically from methane (natural gas). First natural gas is treated to remove sulfur-containing compo ...
... Haber-Bosch Process: A technique for making ammonia from hydrogen and nitrogen, according to the first equation. To get the reactants, nitrogen gas is liquefied form air and hydrogen gas is obtained chemically from methane (natural gas). First natural gas is treated to remove sulfur-containing compo ...
Big Formulas
... As sunlight penetrates into the stratosphere, high-energy UV photons react with oxygen gas molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangement ...
... As sunlight penetrates into the stratosphere, high-energy UV photons react with oxygen gas molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangement ...
I Must Have That Formula APES Chemistry Review From Kelly A
... fires, and lightning produce sulfur dioxide, sulfur trioxide, and nitrogen dioxide. These gases can react with atmospheric water in much the same way that carbon dioxide does to produce sulfurous acid, sulfuric acid, nitric acid and nitrous acid. Ozone Formation and Destruction O2 high-energy UVph ...
... fires, and lightning produce sulfur dioxide, sulfur trioxide, and nitrogen dioxide. These gases can react with atmospheric water in much the same way that carbon dioxide does to produce sulfurous acid, sulfuric acid, nitric acid and nitrous acid. Ozone Formation and Destruction O2 high-energy UVph ...
I Must Have That Formula
... however, high energy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their C-Cl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone by converting it to diatomic oxygen. Every chlorine radical that participat ...
... however, high energy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their C-Cl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone by converting it to diatomic oxygen. Every chlorine radical that participat ...
Document
... As sunlight penetrates into the stratosphere, high-energy UV photons react with oxygen gas molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangement ...
... As sunlight penetrates into the stratosphere, high-energy UV photons react with oxygen gas molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangement ...
I_Must_Have_That_Formula[1]
... equation represents the key ingredients and products of photochemical smog. Hydrocarbons (including VOC’s), carbon monoxide, and nitrogen oxides from vehicle exhausts are irradiated by sunlight in the presence of oxygen gas. The resulting reactions produce a potentially dangerous mixture that includ ...
... equation represents the key ingredients and products of photochemical smog. Hydrocarbons (including VOC’s), carbon monoxide, and nitrogen oxides from vehicle exhausts are irradiated by sunlight in the presence of oxygen gas. The resulting reactions produce a potentially dangerous mixture that includ ...
Sulfur - SOIL 5813
... synthesis of other metabolites, including CoA, biotin, thiamine, and glutathione; main function in proteins is the formation of disulfide bonds between polypeptide chains; component of other S-containing substances, including S-adenosylmethionine, formylmethionine, lipoic acid, and sulfolipid; about ...
... synthesis of other metabolites, including CoA, biotin, thiamine, and glutathione; main function in proteins is the formation of disulfide bonds between polypeptide chains; component of other S-containing substances, including S-adenosylmethionine, formylmethionine, lipoic acid, and sulfolipid; about ...
I Must Have That Formula
... nitrogen oxides from vehicle exhausts are irradiated by sunlight in the presence of oxygen gas. The resulting reactions produce a potentially dangerous mixture that include other nitrogen oxides, ozone, and irritating organic compounds, as well as carbon dioxide and water vapor. Air Pollution Contro ...
... nitrogen oxides from vehicle exhausts are irradiated by sunlight in the presence of oxygen gas. The resulting reactions produce a potentially dangerous mixture that include other nitrogen oxides, ozone, and irritating organic compounds, as well as carbon dioxide and water vapor. Air Pollution Contro ...
Chemistry Review - Woodlawn School Wiki
... solution potassium sulfate and a precipitate fell out. Using balanced chemical equations, show work to find out what ion or ions were in my solution. 2) A 1.42-g sample of a pure compound, with formula M2SO4 , was dissolved in a water and treated with an excess of aqueous barium chloride, resulting ...
... solution potassium sulfate and a precipitate fell out. Using balanced chemical equations, show work to find out what ion or ions were in my solution. 2) A 1.42-g sample of a pure compound, with formula M2SO4 , was dissolved in a water and treated with an excess of aqueous barium chloride, resulting ...
Sulfur dioxide
Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula SO2. At standard atmosphere, it is a toxic gas with a pungent, irritating, and rotten smell. The triple point is 197.69 K and 1.67 kPa. It is released naturally by volcanic activity. Sulfur dioxide was used by the Romans in winemaking when they discovered that burning sulfur candles inside empty wine vessels kept them fresh and free from vinegar smell.



















![I_Must_Have_That_Formula[1]](http://s1.studyres.com/store/data/000170703_1-9524811e6570bb070018bb76b27ae172-300x300.png)