Unit_Chemistry_1a_Oil
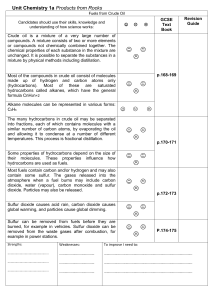
... The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. Some properties of hydrocarbo ...
... The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. Some properties of hydrocarbo ...
Quiz 2
... Quiz 2 Key 1. The EPA limit for CO is 9 ppm. Express this number as a percentage. A. 90% B. 9% C. 0.09% D. 0.0009% Percent is parts per hundred. One hundred is 10,000 times less than one million. 2. The burning of coal produces sulfur dioxide, SO2, a pollutant that slowly reacts in air to form SO3. ...
... Quiz 2 Key 1. The EPA limit for CO is 9 ppm. Express this number as a percentage. A. 90% B. 9% C. 0.09% D. 0.0009% Percent is parts per hundred. One hundred is 10,000 times less than one million. 2. The burning of coal produces sulfur dioxide, SO2, a pollutant that slowly reacts in air to form SO3. ...
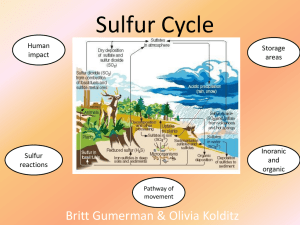
Sulfur dioxide
Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula SO2. At standard atmosphere, it is a toxic gas with a pungent, irritating, and rotten smell. The triple point is 197.69 K and 1.67 kPa. It is released naturally by volcanic activity. Sulfur dioxide was used by the Romans in winemaking when they discovered that burning sulfur candles inside empty wine vessels kept them fresh and free from vinegar smell.