The chemical elements are fundamental building materials of matter
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
1 - Livonia Public Schools
... A) The importance of the equation E = mc2 is that energy has mass. B) Electromagnetic radiation can be thought of as a stream of particles called ...
... A) The importance of the equation E = mc2 is that energy has mass. B) Electromagnetic radiation can be thought of as a stream of particles called ...
8th Grade Post Physical Science Test Study Guide PS 1: The
... B. Compounds are 2 or more elements that are chemically combined. Example of elements – B, Na, C, Cl. Example of compounds - NaCl, KCl, KI, NH3 Element Symbols have either 1 capital letter or 1 capital letter and a lower case letter so if it is a compound there will be 2 or more capital letters. B ...
... B. Compounds are 2 or more elements that are chemically combined. Example of elements – B, Na, C, Cl. Example of compounds - NaCl, KCl, KI, NH3 Element Symbols have either 1 capital letter or 1 capital letter and a lower case letter so if it is a compound there will be 2 or more capital letters. B ...
Quantum mechanics of light dispersion: does the photon have mass?
... each possess the same momentum, then quantum mechanics can only say that they are no more likely to be found in one location than in any other at any given time. A snapshot taken of a large ensemble of such entities will always appear the same, even though one knows that from one moment to the next ...
... each possess the same momentum, then quantum mechanics can only say that they are no more likely to be found in one location than in any other at any given time. A snapshot taken of a large ensemble of such entities will always appear the same, even though one knows that from one moment to the next ...
feb24
... radiation causes those charges to oscillate up and down. In the case of the long wavelength radiation the charge at right oscillates slowly. This is low frequency and low energy motion. The short wavelength causes the charge at right to oscillate more rapidly - high frequency and high energy. These ...
... radiation causes those charges to oscillate up and down. In the case of the long wavelength radiation the charge at right oscillates slowly. This is low frequency and low energy motion. The short wavelength causes the charge at right to oscillate more rapidly - high frequency and high energy. These ...
Michael_Chau_Laeer_Telecomunication_Report
... The concept of a laser involves the amplification of light into an intense ray consisting of photons. These particles have the same direction, frequency, and polarization as well as identical or a phased with a fixed differential with respect to other photons. Because of these attributes, the beam i ...
... The concept of a laser involves the amplification of light into an intense ray consisting of photons. These particles have the same direction, frequency, and polarization as well as identical or a phased with a fixed differential with respect to other photons. Because of these attributes, the beam i ...
2002 Final Exam for Practice - Department of Chemistry | Oregon
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a basic calculator (for example, TI-25X Solar or TI-30XA) if you wish. If you have notes or electronic devices with you, place them in a sealed backpack and p ...
... Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a basic calculator (for example, TI-25X Solar or TI-30XA) if you wish. If you have notes or electronic devices with you, place them in a sealed backpack and p ...
qp2
... The De Broglie hypothesis implied that the distances at which the electron could be found would be where it could form a closed circular standing wave, where the ends meet up as if in a perfect circle. In such a case, the circumference would have to be an integer multiple of the electrons wavelength ...
... The De Broglie hypothesis implied that the distances at which the electron could be found would be where it could form a closed circular standing wave, where the ends meet up as if in a perfect circle. In such a case, the circumference would have to be an integer multiple of the electrons wavelength ...
Chem 1a Midterm Review
... Look at pictures of the hydrogen orbitals at http://www.shef.ac.uk/chemistry/orbitron/ . Note particularly the shape, nodes and sign of the orbitals. Note that the s and d orbitals are symmetric to inversion through the origin while the p is anti-symmetric toward inversions. Orbital energy: one ele ...
... Look at pictures of the hydrogen orbitals at http://www.shef.ac.uk/chemistry/orbitron/ . Note particularly the shape, nodes and sign of the orbitals. Note that the s and d orbitals are symmetric to inversion through the origin while the p is anti-symmetric toward inversions. Orbital energy: one ele ...
Chapter 8 Study Guide
... ν = 5.08 x 1026 1/s b. E = hν E = (6.626 x 10-34 J s) (5.08 x 1026 1/s) = 3.37 x 10-7 J 22. Describe the mechanics and lessons of the glass discharge tubes we looked at with the diffraction glasses. An electron in a hydrogen atom can move between only certain energy states, shown as n = 1 to n = 7. ...
... ν = 5.08 x 1026 1/s b. E = hν E = (6.626 x 10-34 J s) (5.08 x 1026 1/s) = 3.37 x 10-7 J 22. Describe the mechanics and lessons of the glass discharge tubes we looked at with the diffraction glasses. An electron in a hydrogen atom can move between only certain energy states, shown as n = 1 to n = 7. ...
Unit C POS Checklist
... compare and contrast the constituents of the electromagnetic spectrum on the basis of frequency and wavelength. explain the propagation of EMR in terms of perpendicular electric and magnetic fields that are varying with time and travelling away from their source at the speed of light. explain, ...
... compare and contrast the constituents of the electromagnetic spectrum on the basis of frequency and wavelength. explain the propagation of EMR in terms of perpendicular electric and magnetic fields that are varying with time and travelling away from their source at the speed of light. explain, ...
PHYS 2100 Final Examination Introduction to Methods of Theoretical Physics Fall 1998
... You have three hours to complete this exam. There are a total of seven problems. Solve as much of them as you can, but the point total is greater than 100, so “extra credit” is possible. Not all the problems are worth the same number of points. You may use your textbooks and class notes and handouts ...
... You have three hours to complete this exam. There are a total of seven problems. Solve as much of them as you can, but the point total is greater than 100, so “extra credit” is possible. Not all the problems are worth the same number of points. You may use your textbooks and class notes and handouts ...
Quantum Theory of the Atom
... B. Orbitals – describes the electron’s location 1. 2 electrons per orbital 2. s sublevel has 1 orbital (2 electrons total) 3. p sublevel has 3 orbital (6 electrons total) 4. d sublevel has 5 orbital (10 electrons total ) 5. f sublevel has 7 orbital (14 electrons total) Looking at the periodic table ...
... B. Orbitals – describes the electron’s location 1. 2 electrons per orbital 2. s sublevel has 1 orbital (2 electrons total) 3. p sublevel has 3 orbital (6 electrons total) 4. d sublevel has 5 orbital (10 electrons total ) 5. f sublevel has 7 orbital (14 electrons total) Looking at the periodic table ...
The Modern Atomic Model
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
neutrino_trans1
... enough to resolve the oscillations, this guarantees that the wavepackets of the different i still overlap (barely). On the other hand, if the detector energy resolution is poor, and the oscillations can’t be resolved in the energy spectrum, the quantum description of this is that the i have “decoh ...
... enough to resolve the oscillations, this guarantees that the wavepackets of the different i still overlap (barely). On the other hand, if the detector energy resolution is poor, and the oscillations can’t be resolved in the energy spectrum, the quantum description of this is that the i have “decoh ...
Quantum Physics 3 - FSU Physics Department
... photons, i.e. cannot be outcome of destructive or constructive combination of photons interference pattern is due to some inherent property of each photon – it “interferes with itself” while passing from source to screen photons don’t “split” – light detectors always show signals of same intensity ...
... photons, i.e. cannot be outcome of destructive or constructive combination of photons interference pattern is due to some inherent property of each photon – it “interferes with itself” while passing from source to screen photons don’t “split” – light detectors always show signals of same intensity ...
Chapter 7: ELECTRONS IN ATOMS AND
... The student can stop only at certain points on a flight of stairs. Her distance from the ground is quantized. ...
... The student can stop only at certain points on a flight of stairs. Her distance from the ground is quantized. ...
Modern Model of the Atom
... Each energy sublevel corresponds to an ATOMIC ORBITAL (often referred to as a cloud) ...
... Each energy sublevel corresponds to an ATOMIC ORBITAL (often referred to as a cloud) ...
Schr dinger Equation
... but if they are accepted then what follows bears out in the real world. As such QM offers a tool to predict things in chemistry (ionization energy, bond lengths, dipole moments, bond strengths,…) that can be measured in the lab. When these prediction turn out to be accurate we accept that the machin ...
... but if they are accepted then what follows bears out in the real world. As such QM offers a tool to predict things in chemistry (ionization energy, bond lengths, dipole moments, bond strengths,…) that can be measured in the lab. When these prediction turn out to be accurate we accept that the machin ...
Honors Chemistry Week-At-A-Glance
... Review the basic structure of the atom Review calculation atomic mass Calculate wavelength, frequency, and energy of light emitted from atoms Formative Assessment: History, Discriminate between particles, and Atomic mass Quiz Partner Practice: Wavelength, frequency, energy, and electromagnetic ...
... Review the basic structure of the atom Review calculation atomic mass Calculate wavelength, frequency, and energy of light emitted from atoms Formative Assessment: History, Discriminate between particles, and Atomic mass Quiz Partner Practice: Wavelength, frequency, energy, and electromagnetic ...
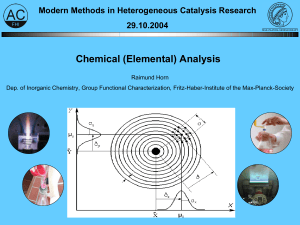
Chemical (Elemental) Analysis - Fritz-Haber
... Ø principle of the standard addition method: - measurement of the analysis sample - stepwise addition of analyte (x+=xA…4xA) - evaluation of xA by extrapolation of the calibration function to the intercept point with the x-axis (y= ...
... Ø principle of the standard addition method: - measurement of the analysis sample - stepwise addition of analyte (x+=xA…4xA) - evaluation of xA by extrapolation of the calibration function to the intercept point with the x-axis (y= ...
Lyman alpha forest
... during the most of the cosmic time from the reionization era. Important probe for cosmic chemical evolution May contain some dust. ...
... during the most of the cosmic time from the reionization era. Important probe for cosmic chemical evolution May contain some dust. ...
Chemistry I Review - BarbaraElam-Rice
... 67) 1 mole of any gas at STP equals ______________. 68) How many grams of ammonium chloride are contained in 0.5 L of a 2 M solution? 69) What is the molarity of a solution on KNO3 that contains 404 grams of KNO3 in 2L of solution? 70) What is the molarity of a solution containing 20 grams of sodium ...
... 67) 1 mole of any gas at STP equals ______________. 68) How many grams of ammonium chloride are contained in 0.5 L of a 2 M solution? 69) What is the molarity of a solution on KNO3 that contains 404 grams of KNO3 in 2L of solution? 70) What is the molarity of a solution containing 20 grams of sodium ...
The Spring 2006 Qualifying Exam, Part 1
... experiments) causes electrons to be emitted. Experiments document the following properties: 1. Electrons are emitted almost instantaneously when light shines on the surface, even for very small light intensities. 2. The rate of photoelectron emission is proportional to the light intensity but does n ...
... experiments) causes electrons to be emitted. Experiments document the following properties: 1. Electrons are emitted almost instantaneously when light shines on the surface, even for very small light intensities. 2. The rate of photoelectron emission is proportional to the light intensity but does n ...
Tailoring single-photon and multiphoton
... need for light sources that produce individual photons. It is of particular importance that these single photons be produced in as controlled a manner as possible, as unwanted additional photons can render quantum cryptographic links insecure and degrade quantum computation efficiencies 关3兴. Single ...
... need for light sources that produce individual photons. It is of particular importance that these single photons be produced in as controlled a manner as possible, as unwanted additional photons can render quantum cryptographic links insecure and degrade quantum computation efficiencies 关3兴. Single ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.