reciprocal lattice g
... quantum mechanical magnetic moment In classical mechanics the Lorentz force is ...
... quantum mechanical magnetic moment In classical mechanics the Lorentz force is ...
constructive - Purdue Physics
... Application: To separate the color of an incident light. The same effect can be obtained with a prism, however a prism is a thick (triangular section) slab of glass and absorbs too much light for certain applications (for example star light). The spectrum of distant stars can be obtained by using a ...
... Application: To separate the color of an incident light. The same effect can be obtained with a prism, however a prism is a thick (triangular section) slab of glass and absorbs too much light for certain applications (for example star light). The spectrum of distant stars can be obtained by using a ...
Topic 14
... Since, in this case the particle is confined by INFINITE potential barriers, we know particle must be located between x=0 and x=L →Normalisation condition reduces to : ...
... Since, in this case the particle is confined by INFINITE potential barriers, we know particle must be located between x=0 and x=L →Normalisation condition reduces to : ...
The Quantum Atom (section 18)
... Thomson proposes plum pudding model. Diffuse positive charge with embedded electrons. Rutherford, Geiger and Marsden experiment (Muncaster p762) fired alpha particles at a thin gold foil. A few alpha particles are deflected through a large angle. Conclusion – there is a small, dense, positively char ...
... Thomson proposes plum pudding model. Diffuse positive charge with embedded electrons. Rutherford, Geiger and Marsden experiment (Muncaster p762) fired alpha particles at a thin gold foil. A few alpha particles are deflected through a large angle. Conclusion – there is a small, dense, positively char ...
Homework Set 1
... c. Now show that, although x(t) and p(t) clearly vary with time, the energy function H[x(t), p(t)] is actually a constant, i.e., a conserved quantity. Do this by plugging in the solutions for x(t) and p(t) found above into the function for H(x, p). Culture: The continuum of energy levels allowed by ...
... c. Now show that, although x(t) and p(t) clearly vary with time, the energy function H[x(t), p(t)] is actually a constant, i.e., a conserved quantity. Do this by plugging in the solutions for x(t) and p(t) found above into the function for H(x, p). Culture: The continuum of energy levels allowed by ...
ATOMIC STRUCTURE Chapter 7
... The new atom laser emits pulses of coherent atoms, or atoms that "march in lock-step." Each pulse contains several million coherent atoms and is accelerated downward by gravity. The curved shape of the pulses was caused by gravity and forces between the atoms. (Field of view 2.5 mm X 5.0 mm.) ...
... The new atom laser emits pulses of coherent atoms, or atoms that "march in lock-step." Each pulse contains several million coherent atoms and is accelerated downward by gravity. The curved shape of the pulses was caused by gravity and forces between the atoms. (Field of view 2.5 mm X 5.0 mm.) ...
Intro to EMR and Wave Equation
... e. light and other EMR consist of oscillating electric and magnetic fields moving together ...
... e. light and other EMR consist of oscillating electric and magnetic fields moving together ...
Uncertainty not so certain after all Early formulation
... Review Letters. “It’s really just this [one aspect] that needs to be updated.” In its most famous articulation, Heisenberg’s uncertainty principle states that it’s possible at a given moment to know either the position or momentum of a particle, but not both. This relationship can be written out mat ...
... Review Letters. “It’s really just this [one aspect] that needs to be updated.” In its most famous articulation, Heisenberg’s uncertainty principle states that it’s possible at a given moment to know either the position or momentum of a particle, but not both. This relationship can be written out mat ...
Waves, particles and fullerenes - Physics | Oregon State University
... passes through one or other of them; but an essential consequence of such a measurement is that the interference pattern is no longer formed, so that the wave properties are no longer manifest. Results such as these led Niels Bohr to propose that the type of properties (particle or wave, for example ...
... passes through one or other of them; but an essential consequence of such a measurement is that the interference pattern is no longer formed, so that the wave properties are no longer manifest. Results such as these led Niels Bohr to propose that the type of properties (particle or wave, for example ...
Physics Qualifier Part I—Spring 2010 7-Minute Questions α
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
... where ωpe is the electron plasma frequency, and γ describes the dissipative processes, γ ≪ ω. Assume that the permeability of the plasma is µ ≈ µ0 , and that ωpe ≪ ω. Estimate the distance such a wave can propagate inside the plasma before it gets significantly attenuated. 7. It is well-known that t ...
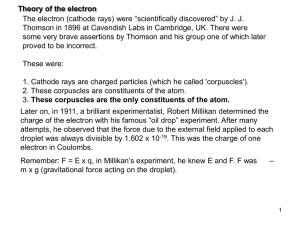
electron_theory
... discrete energy levels for hydrogen. And it worked ! Louis De Broglie then came up with the “wave-particle duality” interpretation for electrons (same for photons). ...
... discrete energy levels for hydrogen. And it worked ! Louis De Broglie then came up with the “wave-particle duality” interpretation for electrons (same for photons). ...
Electromagnetic Radiation
... that are not absorbed by an atom. This is a continuous spectrum with wavelengths removed that are absorbed by the atom. These are shown as black lines for absorbed light. Continuous Spectrum: All wavelengths of a region of the spectrum are represented (i.e. visible light) ...
... that are not absorbed by an atom. This is a continuous spectrum with wavelengths removed that are absorbed by the atom. These are shown as black lines for absorbed light. Continuous Spectrum: All wavelengths of a region of the spectrum are represented (i.e. visible light) ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... obtaining a formal solution of the Schroedinger wave equation in scattering theory. 14) Discuss the time-dependent dependent perturbation theory to obtain an expression for the amplitude of first order transition. 15) Establish that the total angular momentum (orbital + spin) of the electron is a co ...
... obtaining a formal solution of the Schroedinger wave equation in scattering theory. 14) Discuss the time-dependent dependent perturbation theory to obtain an expression for the amplitude of first order transition. 15) Establish that the total angular momentum (orbital + spin) of the electron is a co ...
Light and Energy AP Style
... What’s possible? You can only have a standing wave if you have complete waves. There are only certain allowed waves. In the atom there are certain allowed waves called electrons. ...
... What’s possible? You can only have a standing wave if you have complete waves. There are only certain allowed waves. In the atom there are certain allowed waves called electrons. ...
Zealey Phys-in-Cont
... Electromagnetic Waves A Modern View of Electromagnetic Radiation The wave description of light is most useful in describing the way in which electromagnetic disturbances propagate and interact. It can explain the phenomena of reflection, refraction, diffraction and interference. Electromagnetic wave ...
... Electromagnetic Waves A Modern View of Electromagnetic Radiation The wave description of light is most useful in describing the way in which electromagnetic disturbances propagate and interact. It can explain the phenomena of reflection, refraction, diffraction and interference. Electromagnetic wave ...
Quantum numbers
... Bohr’s model was Imperfect The model of an electron in a circular orbit around a nucleus worked only for Hydrogen, Lithium but by Boron, the model was ineffective. ...
... Bohr’s model was Imperfect The model of an electron in a circular orbit around a nucleus worked only for Hydrogen, Lithium but by Boron, the model was ineffective. ...
PHYSICS CHAPTER 12 NOTES THE NATURE OF LIGHT
... Photon Energy ( h f ) , if it is totally reflected the pressure or change in ...
... Photon Energy ( h f ) , if it is totally reflected the pressure or change in ...
New analysis shows a way to self
... particles, such as electrons, in terms of a wave structure. (In quantum mechanics, waves and particles are considered to be two aspects of the same physical phenomena). By manipulating the wave structure, the team found, it should be possible to cause electrons to behave in unusual and counterintuit ...
... particles, such as electrons, in terms of a wave structure. (In quantum mechanics, waves and particles are considered to be two aspects of the same physical phenomena). By manipulating the wave structure, the team found, it should be possible to cause electrons to behave in unusual and counterintuit ...