WHAT IS NOETHER`S THEOREM? - Ohio State Department of
... Both of these are examples of a much more general phenomena; for every conserved quantity there is an associated differentiable symmetry. This holds in great generality.. Exercise 1. Find the associated symmetry associated to conservation of angular momentum and derive conservation of angular moment ...
... Both of these are examples of a much more general phenomena; for every conserved quantity there is an associated differentiable symmetry. This holds in great generality.. Exercise 1. Find the associated symmetry associated to conservation of angular momentum and derive conservation of angular moment ...
Triadic Quantum Energy
... economy , so that the long range conceptual interactions between artist ad scientists go down opening the technological frontier of productive advancements , so that art and science, during this period , both remains closed in proper disciplinary domain. Today’s the “Quantum E ...
... economy , so that the long range conceptual interactions between artist ad scientists go down opening the technological frontier of productive advancements , so that art and science, during this period , both remains closed in proper disciplinary domain. Today’s the “Quantum E ...
Matter–wave interference of particles selected from a molecular
... interferes only with itself. We only have to make sure that every molecule contributes to the final pattern in a similar way, which is true for all members of the library with about the same mass, independent of their internal state. Differences between various molecules, such as their isotopic distr ...
... interferes only with itself. We only have to make sure that every molecule contributes to the final pattern in a similar way, which is true for all members of the library with about the same mass, independent of their internal state. Differences between various molecules, such as their isotopic distr ...
fermi dirac statistics in solids
... i.e. the result for electrical conductivity must be one order of magnitude too small, which is observed !! But L for particle is quite reasonable, so replace Vrms with Vfermi and the conductivity gets one order of magnitude larger, which is close to the experimental observation, so that one keeps th ...
... i.e. the result for electrical conductivity must be one order of magnitude too small, which is observed !! But L for particle is quite reasonable, so replace Vrms with Vfermi and the conductivity gets one order of magnitude larger, which is close to the experimental observation, so that one keeps th ...
slides
... introduce two azimuthal quantum numbers and then in talking about the s, p, d separation you use one and in talking about the fine structure splitting you use the other. And the question is how can you have two quantum numbers that describe the eccentricity of the orbit? It only has one eccentricity ...
... introduce two azimuthal quantum numbers and then in talking about the s, p, d separation you use one and in talking about the fine structure splitting you use the other. And the question is how can you have two quantum numbers that describe the eccentricity of the orbit? It only has one eccentricity ...
Chapter 7 The Quantum- Mechanical Model of the Atom - NTOU-Chem
... It was observed that many metals emit electrons when a light shines on their surface. – This is called the photoelectric effect. Classic wave theory attributed this effect to the light energy being transferred to the electron. According to this theory, if the wavelength of light is made shorter, or ...
... It was observed that many metals emit electrons when a light shines on their surface. – This is called the photoelectric effect. Classic wave theory attributed this effect to the light energy being transferred to the electron. According to this theory, if the wavelength of light is made shorter, or ...
Academic Physics Semester II Review Sheet
... 10. A student touches an electroscope with his hand at the same time he brings a positively charged rod close to the electroscope without touching. When he removes his hand first and then moves the rod away from the electroscope the leaves move apart. Why? What type of charge is on the leaves? 11. T ...
... 10. A student touches an electroscope with his hand at the same time he brings a positively charged rod close to the electroscope without touching. When he removes his hand first and then moves the rod away from the electroscope the leaves move apart. Why? What type of charge is on the leaves? 11. T ...
Sects. 2.6 & 2.7
... • Implied assumptions of Newtonian Mechanics: – r, v, t, p, E are all measurable (simultaneously!) – All can be specified with desired accuracy, depending only on the sophistication of our measuring instruments. True for MACROSCOPIC objects! Not true for MICROSCOPIC (atomic & smaller) objects! – Qua ...
... • Implied assumptions of Newtonian Mechanics: – r, v, t, p, E are all measurable (simultaneously!) – All can be specified with desired accuracy, depending only on the sophistication of our measuring instruments. True for MACROSCOPIC objects! Not true for MICROSCOPIC (atomic & smaller) objects! – Qua ...
Another version - Scott Aaronson
... A Few Other Things I’ve Worked On The limitations of quantum computers (e.g., for finding collisions in hash functions); the possibility of quantum-secure cryptography What’s the largest possible quantum speedup? (The Forrelation and k-fold Forrelation problems) Quantum computing and the black-hole ...
... A Few Other Things I’ve Worked On The limitations of quantum computers (e.g., for finding collisions in hash functions); the possibility of quantum-secure cryptography What’s the largest possible quantum speedup? (The Forrelation and k-fold Forrelation problems) Quantum computing and the black-hole ...
The stability of an atom depends on the ratio and number of protons
... Most odd-odd nuclei are highly unstable with respect to beta decay because the decay products are even-even and therefore more strongly bound, due to nuclear pairing effects. An atom with an unstable nucleus, called a radionuclide, is characterized by excess energy available either for a newly crea ...
... Most odd-odd nuclei are highly unstable with respect to beta decay because the decay products are even-even and therefore more strongly bound, due to nuclear pairing effects. An atom with an unstable nucleus, called a radionuclide, is characterized by excess energy available either for a newly crea ...
The angular momentum quantum number
... quantum mechanical model. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms. A model is useful because it helps you understand what’s observed in nature. It’s not unusual ...
... quantum mechanical model. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms. A model is useful because it helps you understand what’s observed in nature. It’s not unusual ...
here - Dalibor Hrg
... Turing machine, quantum class (BQP) and first quantum algorithm (Deutsch-Jozsa). Postulates of quantum mechanics, superposition of states, interference, unitary operators on Hilbert space, tensorial calculation,… ...
... Turing machine, quantum class (BQP) and first quantum algorithm (Deutsch-Jozsa). Postulates of quantum mechanics, superposition of states, interference, unitary operators on Hilbert space, tensorial calculation,… ...
The roads not taken: empty waves, wavefunction collapse and
... makes reference to ‘particle’ properties such as mass, the linearly evolving wavefunction ψ ( x ) does not generally exhibit any feature that could be put into correspondence with a localized particle structure. To turn quantum mechanics into a theory of matter and motion, with real atoms and molecu ...
... makes reference to ‘particle’ properties such as mass, the linearly evolving wavefunction ψ ( x ) does not generally exhibit any feature that could be put into correspondence with a localized particle structure. To turn quantum mechanics into a theory of matter and motion, with real atoms and molecu ...
Lecture 9
... The reason for the repetition is that quantum mechanics does not make definite predictions for the position, momentum, etc. When we do the exact same measurement on identically prepared systems, we do not get always get the same result, as we do in classical mechanics. But probability distributions ...
... The reason for the repetition is that quantum mechanics does not make definite predictions for the position, momentum, etc. When we do the exact same measurement on identically prepared systems, we do not get always get the same result, as we do in classical mechanics. But probability distributions ...
Name: Date: Chemistry Enriched Per. ______ Midterm Review
... split the logs and let them “season” (dry out) for at least 6 months. You then burn the firewood in your highly efficient, “clean” burning wood stove. Describe the chemical and physical processes taking place…and describe how you know the difference… ...
... split the logs and let them “season” (dry out) for at least 6 months. You then burn the firewood in your highly efficient, “clean” burning wood stove. Describe the chemical and physical processes taking place…and describe how you know the difference… ...
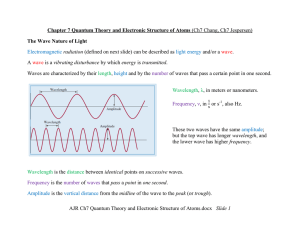
AJR Ch7 Quantum Theory and Electronic Structure of Atoms.docx
... Wave Functions and Quantum Numbers Quantum Mechanics and Atomic Orbitals In 1926, Schrödinger formulated an equation that describes the behavior and energies of submicroscopic particles in general, an equation analogous to Newton’s laws of motion for macroscopic objects. The Schrödinger equation in ...
... Wave Functions and Quantum Numbers Quantum Mechanics and Atomic Orbitals In 1926, Schrödinger formulated an equation that describes the behavior and energies of submicroscopic particles in general, an equation analogous to Newton’s laws of motion for macroscopic objects. The Schrödinger equation in ...
Module P10.2 A wave model for matter
... within physics, embracing as it does all the elements of classical physics as well as the new discoveries. ...
... within physics, embracing as it does all the elements of classical physics as well as the new discoveries. ...