1B11 Foundations of Astronomy Star names and magnitudes
... 1B11 The electromagnetic spectrum When an electric charge is accelerated, electromagnetic energy is produced. This energy can be thought of as propagating as a wave – or, equally as a particle. The waves are usually referred to as light waves or radiation. The particles are known as photons. ...
... 1B11 The electromagnetic spectrum When an electric charge is accelerated, electromagnetic energy is produced. This energy can be thought of as propagating as a wave – or, equally as a particle. The waves are usually referred to as light waves or radiation. The particles are known as photons. ...
ATOMIC STRUCTURE
... En = (-RH)(1/n2) n = 1,2,3,4…. RH = Rydberg constant (2.18 x 10-18 J) n = principle quantun number ...
... En = (-RH)(1/n2) n = 1,2,3,4…. RH = Rydberg constant (2.18 x 10-18 J) n = principle quantun number ...
Quantum Mechanics 1 - University of Birmingham
... To understand wave-particle duality and know the relationships between momentum, frequency, wavelength and energy for “particles” and “waves”. To be able to write down the Schrödinger equation for particles: in a 1D box; in 1- and 2-electron atoms; in 1- and 2-electron molecules. To know the origins ...
... To understand wave-particle duality and know the relationships between momentum, frequency, wavelength and energy for “particles” and “waves”. To be able to write down the Schrödinger equation for particles: in a 1D box; in 1- and 2-electron atoms; in 1- and 2-electron molecules. To know the origins ...
Lecture 15 (Slides) September 28
... H Atom Wave Functions • The previous slide states that the H atom wave functions, determined again by solving the Schrodinger equation, can be factored into an angular and a radial part if we employ spherical polar coordinates. The use of these coordinates makes it especially easy to locate nodes ( ...
... H Atom Wave Functions • The previous slide states that the H atom wave functions, determined again by solving the Schrodinger equation, can be factored into an angular and a radial part if we employ spherical polar coordinates. The use of these coordinates makes it especially easy to locate nodes ( ...
Lecture 5
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
... tells you the probability that a measurement of the energy would yield the value En. Only the values En can be obtained as results of the energy measurements. The sum of all these probabilities will be, of course, 1. (see proof in the textbook) ...
Lab 11
... e. Repeat (1d) for intensity levels 2, 3, and 4. Plot iphoto vs. Vincline for all four intensities on a single plot. f. Using your observations and the plot, answer the questions of (i) time lag, (ii) Vstop (Kmax) ∝ Intensity, and (iii) iphoto ∝ intensity , and based on the predictions of the Wav ...
... e. Repeat (1d) for intensity levels 2, 3, and 4. Plot iphoto vs. Vincline for all four intensities on a single plot. f. Using your observations and the plot, answer the questions of (i) time lag, (ii) Vstop (Kmax) ∝ Intensity, and (iii) iphoto ∝ intensity , and based on the predictions of the Wav ...
33-6 Radiation Pressure
... 33-6 Radiation Pressure Electromagnetic waves have linear momentum as well as energy exerted a radiation pressure on an object by shining light on it. the pressure must be very small like a camera flash every photographic flash could be like a punch. Finding an expression for the press ...
... 33-6 Radiation Pressure Electromagnetic waves have linear momentum as well as energy exerted a radiation pressure on an object by shining light on it. the pressure must be very small like a camera flash every photographic flash could be like a punch. Finding an expression for the press ...
review
... impossible, as it violated the local realist view of causality (Einstein referred to it as "spooky action at a distance"),[4] and argued that the accepted formulation of quantum mechanics must therefore be incomplete. Later, however, the counterintuitive predictions of quantum mechanics were verifie ...
... impossible, as it violated the local realist view of causality (Einstein referred to it as "spooky action at a distance"),[4] and argued that the accepted formulation of quantum mechanics must therefore be incomplete. Later, however, the counterintuitive predictions of quantum mechanics were verifie ...
The Quantum Mechanical Model
... Determine the de Broglie wavelength for an electron moving at a speed of 9. x 106m/s. (me= 9.1 x 10 -31 kg) Answer: 8.09 x 10 -11 m ...
... Determine the de Broglie wavelength for an electron moving at a speed of 9. x 106m/s. (me= 9.1 x 10 -31 kg) Answer: 8.09 x 10 -11 m ...
1 The Photoelectric Effect 2 Line Spectra and Energy Levels
... where m is the electron mass. Mathematica Demonstrations: Compton Effect ...
... where m is the electron mass. Mathematica Demonstrations: Compton Effect ...
spectral lines
... electrical forces of attraction. Rutherford’s model was very appealing but there were some “minor” problems that had to be solved. What held the nucleus together to be so small? AND… The orbiting electrons were giving off light, due to Conservation of Energy, they should eventually spiral into ...
... electrical forces of attraction. Rutherford’s model was very appealing but there were some “minor” problems that had to be solved. What held the nucleus together to be so small? AND… The orbiting electrons were giving off light, due to Conservation of Energy, they should eventually spiral into ...
Quantum Problems 1. Consider a quantum system whose state at
... 1. Consider a quantum system whose state at time t1 is given by |Ψ(t1 )i = √12 (|ψ1 i + |ψ2 i), where |ψ1 i and |ψ2 i are energy eigenstates with eigenvalues E1 and E2 respectively. (E1 6= E2 .) (a) Calculate the uncertainty ∆E of the system, as well as the first time t2 > t1 at which |Ψ(t2 )i becom ...
... 1. Consider a quantum system whose state at time t1 is given by |Ψ(t1 )i = √12 (|ψ1 i + |ψ2 i), where |ψ1 i and |ψ2 i are energy eigenstates with eigenvalues E1 and E2 respectively. (E1 6= E2 .) (a) Calculate the uncertainty ∆E of the system, as well as the first time t2 > t1 at which |Ψ(t2 )i becom ...
DARLLENWCH Y DARN ISOD AC ATEBWCH Y CWESTIYNAU SY
... In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curv ...
... In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curv ...
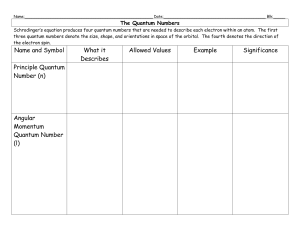
e-the-quantum-numberssv-2
... The Quantum Numbers Schrodinger’s equation produces four quantum numbers that are needed to describe each electron within an atom. The first three quantum numbers denote the size, shape, and orientations in space of the orbital. The fourth denotes the direction of the electron spin. ...
... The Quantum Numbers Schrodinger’s equation produces four quantum numbers that are needed to describe each electron within an atom. The first three quantum numbers denote the size, shape, and orientations in space of the orbital. The fourth denotes the direction of the electron spin. ...
Chapter 1
... But quantum mechanics is stubborn and won’t. Statistically speaking, the cat (goes the joke), Is half a cat breathing and half a cat croaked. To some this may seem a ridiculous split, But quantum mechanics must answer, “Tough @#&! We may not know much, but one thing’s fo’ sho’: There’s things in the ...
... But quantum mechanics is stubborn and won’t. Statistically speaking, the cat (goes the joke), Is half a cat breathing and half a cat croaked. To some this may seem a ridiculous split, But quantum mechanics must answer, “Tough @#&! We may not know much, but one thing’s fo’ sho’: There’s things in the ...