Questions for learning Quantum Mechanics of FYSA21
... Experimental background 1. At the beginning of last century, four important effects/experiments/models that led to problems when analysed within the framework of classical physics were • black body radiation, • Rutherfords model of the atom, • the photoelectric effect, • Compton scattering. Chose two ...
... Experimental background 1. At the beginning of last century, four important effects/experiments/models that led to problems when analysed within the framework of classical physics were • black body radiation, • Rutherfords model of the atom, • the photoelectric effect, • Compton scattering. Chose two ...
lecture 5 radiation and matter
... appear brighter than the organic egg. We do not need to consider the wave phenomena of diffraction and interference when interpreting this image. ...
... appear brighter than the organic egg. We do not need to consider the wave phenomena of diffraction and interference when interpreting this image. ...
Chemistry Ch 4
... Ground state = lowest energy state of an atom Excited state = the highest energy state When atoms are excited by energy (heat), they emit energy in the form of light. ...
... Ground state = lowest energy state of an atom Excited state = the highest energy state When atoms are excited by energy (heat), they emit energy in the form of light. ...
A1979HZ36600001
... that all states with this property (e.g., all states of energy E) can be written as linearcombinations α1 ψ1 + α2 ψ2 + .....of these. “If the specified set of states is invariant under some transformations—as are the states of an atom of definite energy under rotations, the state obtained from one o ...
... that all states with this property (e.g., all states of energy E) can be written as linearcombinations α1 ψ1 + α2 ψ2 + .....of these. “If the specified set of states is invariant under some transformations—as are the states of an atom of definite energy under rotations, the state obtained from one o ...
Schr dinger Equation
... they don’t interfere with themselves) gives rise to quantized solutions. 2. If you combine this with Bohr’s idea of quantize angular momentum you arrive at a relationship between wavelength and linear momentum. ...
... they don’t interfere with themselves) gives rise to quantized solutions. 2. If you combine this with Bohr’s idea of quantize angular momentum you arrive at a relationship between wavelength and linear momentum. ...
“We choose to examine a phenomenon which is impossible
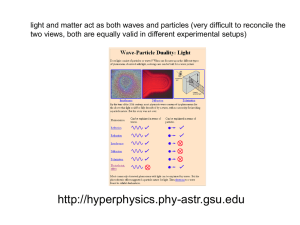
... The important results from last time: Quantum mechanical entities can exhibit either wave-like or particle-like properties, depending on what one measures. We saw this phenomenon for photons, and claimed that it is also true for matter (e.g., electrons). The wave and particle properties are related ...
... The important results from last time: Quantum mechanical entities can exhibit either wave-like or particle-like properties, depending on what one measures. We saw this phenomenon for photons, and claimed that it is also true for matter (e.g., electrons). The wave and particle properties are related ...
Degeneracy of Hydrogen atom
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
Atomic Structure Zumdahl Chemistry Chapter 7
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
11 Applications III
... Blackbody radiation is an extremely important application of statistical mechanics. A heated object radiates energy in the form of photons whose frequency distribution depends on the temperature of the object. Photons are Bose particles and therefore obey the occupation number expression (5). Each p ...
... Blackbody radiation is an extremely important application of statistical mechanics. A heated object radiates energy in the form of photons whose frequency distribution depends on the temperature of the object. Photons are Bose particles and therefore obey the occupation number expression (5). Each p ...
Lecture 2 EMS - San Jose State University
... (in units of cycles per second, whose SI version is Hertz [1 Hertz = 1/s-1]) ...
... (in units of cycles per second, whose SI version is Hertz [1 Hertz = 1/s-1]) ...
7.Spectral Properties
... Colour of Transition metal complexes If λ is wave length of radiation absorbed, its energy E = hc/ λ , where c = velocity of light, h = Planck’s constant. For example [Ti(H2O)6]+. Ti has d1configuration and single electron occupies t2g orbital. When light energy is passed thru its solution it absor ...
... Colour of Transition metal complexes If λ is wave length of radiation absorbed, its energy E = hc/ λ , where c = velocity of light, h = Planck’s constant. For example [Ti(H2O)6]+. Ti has d1configuration and single electron occupies t2g orbital. When light energy is passed thru its solution it absor ...
Wave particle-interactions
... at ‚position‘φ in ‚time‘ x. Via multiplication with ∂φ/∂x and integration with respect to x, we finally obtain: ...
... at ‚position‘φ in ‚time‘ x. Via multiplication with ∂φ/∂x and integration with respect to x, we finally obtain: ...
Section 4.2 The Quantum Model of the Atom
... • Explain how the Heisenberg uncertainty principle and the Schrodinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers, and describe their significance. Electrons Act Like Both Particles and Waves ...
... • Explain how the Heisenberg uncertainty principle and the Schrodinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers, and describe their significance. Electrons Act Like Both Particles and Waves ...
AP Chemistry – Chapter 6 Reading Guide: Electronic Structure of
... 13. Describe what is meant by the s, p, d, and f blocks of the periodic table. Explain where they are found on the periodic table. ...
... 13. Describe what is meant by the s, p, d, and f blocks of the periodic table. Explain where they are found on the periodic table. ...
Quantum and Photo-electric effects - Delivery guide
... Extension activities could include shining a UV lamp onto a zinc plate sitting on a Coulombmeter (see later section) or researching how the CCD in a digital camera works. The following three sections are arguably the most engaging of the A Level, particularly for the students; not because they are f ...
... Extension activities could include shining a UV lamp onto a zinc plate sitting on a Coulombmeter (see later section) or researching how the CCD in a digital camera works. The following three sections are arguably the most engaging of the A Level, particularly for the students; not because they are f ...
Energy and Matter - Hicksville Public Schools
... Electrons related to the atom’s highest principle energy level are referred to as valance electrons. Valence electrons determine the chemical properties of an element. ...
... Electrons related to the atom’s highest principle energy level are referred to as valance electrons. Valence electrons determine the chemical properties of an element. ...
Foundations, 2
... All Newtonian particles have mass. To determine the mass of the photon we borrow one general result from Maxwell and a second general result from special relativity. The result from Maxwell has to do with the fact that EM radiation carries both energy and momentum. Momentum transport in EM waves res ...
... All Newtonian particles have mass. To determine the mass of the photon we borrow one general result from Maxwell and a second general result from special relativity. The result from Maxwell has to do with the fact that EM radiation carries both energy and momentum. Momentum transport in EM waves res ...