On the physical structure of radiant energy: waves and
... The wave theory supported by Huygens, Fresnel and Young claimed light propagates through space by mechanical oscillations of a transmissive medium called ether. Then in the 19th century Maxwell developed a wave electromagnetic theory[1] in which electromagnetic waves propagate with the speed of ligh ...
... The wave theory supported by Huygens, Fresnel and Young claimed light propagates through space by mechanical oscillations of a transmissive medium called ether. Then in the 19th century Maxwell developed a wave electromagnetic theory[1] in which electromagnetic waves propagate with the speed of ligh ...
Physics 124 : Particles and Waves
... The images below show a striking example of this, where electrons are fired, one at a time, toward a double slit the positions of the electrons that make it through the slit and hit the screen are recorded with time, the characteristic double-slit diffraction pattern appears ...
... The images below show a striking example of this, where electrons are fired, one at a time, toward a double slit the positions of the electrons that make it through the slit and hit the screen are recorded with time, the characteristic double-slit diffraction pattern appears ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... 2. Until now we saw that all sigma (circular polatization) signs and/or helicities are the same for counter propagating beams in the MOT. Why is the above picture (also explaining a Mirror MOT) different? Hint: the energy level diagram will also look different, but its just different notation explai ...
... 2. Until now we saw that all sigma (circular polatization) signs and/or helicities are the same for counter propagating beams in the MOT. Why is the above picture (also explaining a Mirror MOT) different? Hint: the energy level diagram will also look different, but its just different notation explai ...
5.11 Harmonic Oscillator
... A truly classic example is the swinging bowling ball demo. Before we continue, let’s think about harmonic oscillators… Classically, all energies are allowed. What will QM say? Only quantized energies? Classically, an energy of zero is allowed. What will QM say? Nonzero, like particle in box? Classi ...
... A truly classic example is the swinging bowling ball demo. Before we continue, let’s think about harmonic oscillators… Classically, all energies are allowed. What will QM say? Only quantized energies? Classically, an energy of zero is allowed. What will QM say? Nonzero, like particle in box? Classi ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... U U 0 n 0 (0) n1 (1 0 ) n 2 ( 2 0 ) ..... - The equation of partition function for the particle at i=0 ...
... U U 0 n 0 (0) n1 (1 0 ) n 2 ( 2 0 ) ..... - The equation of partition function for the particle at i=0 ...
Homework 8 - spacibm configuration notes
... This develops higher order contributions to the motion. Repeat the process to obtain an infinite series for each component to provide an exact description (perturbation expansion) of the electron’s motion in the coherent wave. If you can sum the series analytically, do so. Interpret your results. (e ...
... This develops higher order contributions to the motion. Repeat the process to obtain an infinite series for each component to provide an exact description (perturbation expansion) of the electron’s motion in the coherent wave. If you can sum the series analytically, do so. Interpret your results. (e ...
Helical Particle Waves
... and amplitude is easily understood today. However physicists are still today unable to explain how a stream of photons can behave as both a stream of particles and as a wave at the same time and satisfy themselves by claiming it is a mass when you look for mass and it is a wave when you look for wav ...
... and amplitude is easily understood today. However physicists are still today unable to explain how a stream of photons can behave as both a stream of particles and as a wave at the same time and satisfy themselves by claiming it is a mass when you look for mass and it is a wave when you look for wav ...
king fahd university of petroleum and minerals
... Summary of the main learning outcomes for students enrolled in the course. At the end of this course the student should be able to: 1. Have an ample knowledge about special relativity. 2. Know about the quantum nature of atoms. 3. Solve problems related to quantum mechanics in one, two, and three di ...
... Summary of the main learning outcomes for students enrolled in the course. At the end of this course the student should be able to: 1. Have an ample knowledge about special relativity. 2. Know about the quantum nature of atoms. 3. Solve problems related to quantum mechanics in one, two, and three di ...
Document
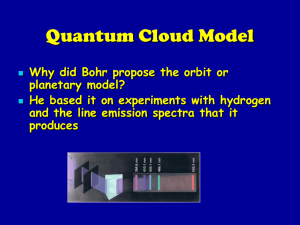
... 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The energy of the electron is greater when it is in orbits farther from the nucleus 3. The atom achieves the ground state when atoms occupy the closest possible positions around the ...
... 1. Electrons can circle the nucleus only in allowed paths or orbits 2. The energy of the electron is greater when it is in orbits farther from the nucleus 3. The atom achieves the ground state when atoms occupy the closest possible positions around the ...
Electrons!
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
PowerPoint
... Picture of the Atom The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers n ...
... Picture of the Atom The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers n ...
The Quantum Mechanical Picture of the Atom
... The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers ...
... The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers ...
energy - U of L Class Index
... Light as a Particle The photoelectric effect. In 1888, Heinrich Hertz discovered that electrons could be ejected from a sample by shining light on it. Note the effects of changing: Intensity - No matter how low the intensity there is still a current?? Frequency - Must have sufficient energy to eject ...
... Light as a Particle The photoelectric effect. In 1888, Heinrich Hertz discovered that electrons could be ejected from a sample by shining light on it. Note the effects of changing: Intensity - No matter how low the intensity there is still a current?? Frequency - Must have sufficient energy to eject ...
Operators and meaning of wave function
... Quantum theory is the theoretical basis of modern physics that explain behavior of matter on the atomic and subatomic level. Interpretation of quantum theory deals with two problems: how to relate the mathematical formalism of quantum theory to empirical observations, and how to understand that rela ...
... Quantum theory is the theoretical basis of modern physics that explain behavior of matter on the atomic and subatomic level. Interpretation of quantum theory deals with two problems: how to relate the mathematical formalism of quantum theory to empirical observations, and how to understand that rela ...
5 Electrons in Atoms
... preceding ruthenium is krypton (Kr), which has an atomic number of 36. Represent ruthenium's first 36 electrons using the chemical ...
... preceding ruthenium is krypton (Kr), which has an atomic number of 36. Represent ruthenium's first 36 electrons using the chemical ...
stationary state
... From grid to plate, this process could happen multiple times, therefore a periodical reduction in current is observed. ...
... From grid to plate, this process could happen multiple times, therefore a periodical reduction in current is observed. ...
Extension physics
... Find the frequency and describe the normal modes of a given coupled oscillator. Waves and Electromagnetic Radiation After studying this section pupils should be able to… Recall the wave equation for a propagating wave. Show by substitution into the wave equation that expressions of the form ei(tkx ...
... Find the frequency and describe the normal modes of a given coupled oscillator. Waves and Electromagnetic Radiation After studying this section pupils should be able to… Recall the wave equation for a propagating wave. Show by substitution into the wave equation that expressions of the form ei(tkx ...