Nonlincourse13
... The similarity to the classical case is reassuring. Only off-diagonal ("nonresonant") terms can be eliminated by a nonsingular transformation. The resulting Hamiltonian is diagonal, but nonlinear. The generator of the transformation is determined up to a diagonal ("resonant") term. This procedure ca ...
... The similarity to the classical case is reassuring. Only off-diagonal ("nonresonant") terms can be eliminated by a nonsingular transformation. The resulting Hamiltonian is diagonal, but nonlinear. The generator of the transformation is determined up to a diagonal ("resonant") term. This procedure ca ...
Quantum Technologies - Connect Innovate UK
... CompeJJon for Industry-‐lead QT projects • ‘Exploring the commercial applicaJons of quantum technologies’ ...
... CompeJJon for Industry-‐lead QT projects • ‘Exploring the commercial applicaJons of quantum technologies’ ...
Periodic Boundary Conditions. Classical Limit ( + problems 27
... localized wave packets of the size much larger than λT , but much smaller than L. On one hand, ...
... localized wave packets of the size much larger than λT , but much smaller than L. On one hand, ...
Bohr Model of the Atom
... changing energy states, a photon would be emitted with energy equal to that change Bohr (1913) argued that perhaps electrons in the atom may also behave in this way. Electrons don’t radiate with just any energy, but rather must do so in a quantised fashion. He developed the following theory of the a ...
... changing energy states, a photon would be emitted with energy equal to that change Bohr (1913) argued that perhaps electrons in the atom may also behave in this way. Electrons don’t radiate with just any energy, but rather must do so in a quantised fashion. He developed the following theory of the a ...
Lecture 9
... for one electron atoms, but failed completely for atoms with more than one electron! Something was still missing! ...
... for one electron atoms, but failed completely for atoms with more than one electron! Something was still missing! ...
Document
... In mechanics and field theory (both classical and quantum), there are two main languages - Lagrangian and Hamiltonian. In the classical setting, the Lagrangian language is the language of vari-ational calculus (i.e. one studies extremals of the action functional), while the Hamiltonian language is t ...
... In mechanics and field theory (both classical and quantum), there are two main languages - Lagrangian and Hamiltonian. In the classical setting, the Lagrangian language is the language of vari-ational calculus (i.e. one studies extremals of the action functional), while the Hamiltonian language is t ...
simulate quantum systems
... appropriate Hamiltonian, and by tuning the delays, it becomes possible to drive the system through the phase transitions. The results are shown in Fig. 2. For our simulation, we chose a system Hamiltonian that avoids degeneracies of the ground state. This allowed us to adiabatically change the Hamil ...
... appropriate Hamiltonian, and by tuning the delays, it becomes possible to drive the system through the phase transitions. The results are shown in Fig. 2. For our simulation, we chose a system Hamiltonian that avoids degeneracies of the ground state. This allowed us to adiabatically change the Hamil ...
Statistical Physics
... The Boltzmann distribution is valid when e eE/kT >> 1. This can occur because of low particle densities and energies >> kT ...
... The Boltzmann distribution is valid when e eE/kT >> 1. This can occur because of low particle densities and energies >> kT ...
Quantum Mechanics - UCSD Department of Physics
... • Every particle or system of particles can be defined in quantum mechanical terms – and therefore have wave-like properties ...
... • Every particle or system of particles can be defined in quantum mechanical terms – and therefore have wave-like properties ...
DARLLENWCH Y DARN ISOD AC ATEBWCH Y CWESTIYNAU SY
... in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit ...
... in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit ...
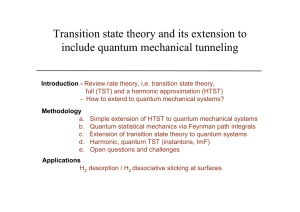
Transition state theory and its extension to include quantum
... “In view of [its] success, it is unfortunate that the theory [TST] does not enjoy a better understanding and confidence among non-specialists. Some of this difficulty can be traced to the rather unconvincing derivations of the [TST] expression for the rate constant which are found in many physical c ...
... “In view of [its] success, it is unfortunate that the theory [TST] does not enjoy a better understanding and confidence among non-specialists. Some of this difficulty can be traced to the rather unconvincing derivations of the [TST] expression for the rate constant which are found in many physical c ...
chapter 7 part 3
... spectral lines, when atom radiates in magnetic field, spacing of the lines depends on magnetic energy,, only variable, is magnetic flux density, B – experimental evidence for space quantization, 1896, could not be explained by Bohr model, 1913 changes in ml are restricted to ml 0 _ or _ 1 by se ...
... spectral lines, when atom radiates in magnetic field, spacing of the lines depends on magnetic energy,, only variable, is magnetic flux density, B – experimental evidence for space quantization, 1896, could not be explained by Bohr model, 1913 changes in ml are restricted to ml 0 _ or _ 1 by se ...
Document
... A hydrogen atom electron is excited to an energy of −13.6/4 eV. How many different quantum states could the electron be in? That is, how many wave functions ynℓm have this energy? ...
... A hydrogen atom electron is excited to an energy of −13.6/4 eV. How many different quantum states could the electron be in? That is, how many wave functions ynℓm have this energy? ...
Scientific Papers
... flask of poison gas. Attach to this scene a radioactive which will decay after a certain amount of time (measured by the half-life of the atom). This will cause the flask to open and set the gas free. Assume that the decay of the atom is completely random - that is, there is a fifty-fifty chance of ...
... flask of poison gas. Attach to this scene a radioactive which will decay after a certain amount of time (measured by the half-life of the atom). This will cause the flask to open and set the gas free. Assume that the decay of the atom is completely random - that is, there is a fifty-fifty chance of ...
Physics 452 - BYU Physics and Astronomy
... To be distributed in the energy levels E1 and E2 What is the number of combinations Q(2, 4, 0,....) ? ...
... To be distributed in the energy levels E1 and E2 What is the number of combinations Q(2, 4, 0,....) ? ...
Physics 228, Lecture 11 Monday, February 28, 2005 Bohr Model
... only by negligible amounts as long as the velocities of particles were much less than the speed of light. In our treatment of quantum mechanical effects, it is less clear in what approximation quantum mechanical effects should be negligible, but we must have that the true physics is well approximate ...
... only by negligible amounts as long as the velocities of particles were much less than the speed of light. In our treatment of quantum mechanical effects, it is less clear in what approximation quantum mechanical effects should be negligible, but we must have that the true physics is well approximate ...
III. Quantum Model of the Atom
... n = # of sublevels per energy level n2 = # of orbitals per energy level Orbitals per sublevel: 1 s, 3 p, 5 d, 7 f ...
... n = # of sublevels per energy level n2 = # of orbitals per energy level Orbitals per sublevel: 1 s, 3 p, 5 d, 7 f ...