Nitrogen source governs the patterns of growth and
... for their ability to sustain high antibiotic titres and this property is supposed to be linked to the slow release of nitrogenous components during the course of the fermentation. More generally, several studies have shown that nitrogen assimilation is crucial for the regulation of antibiotic produc ...
... for their ability to sustain high antibiotic titres and this property is supposed to be linked to the slow release of nitrogenous components during the course of the fermentation. More generally, several studies have shown that nitrogen assimilation is crucial for the regulation of antibiotic produc ...
Full Text Attachment - international journal of advances in
... ho r mo ne , histamine is a vasodilator and is a major factor in allergic reaction such as hay fever [3, 4]. CHEMISTRY OF IMIDAZOLES Imidazole ring has attracted considerable attention in the last 30 years. i.e industry started in the year 1970, regarding its chemistry structure, physical properties ...
... ho r mo ne , histamine is a vasodilator and is a major factor in allergic reaction such as hay fever [3, 4]. CHEMISTRY OF IMIDAZOLES Imidazole ring has attracted considerable attention in the last 30 years. i.e industry started in the year 1970, regarding its chemistry structure, physical properties ...
The p-Block Elements The p-Block Elements
... pπ -p π multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p π -pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a dia ...
... pπ -p π multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p π -pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a dia ...
Role of Amino Acids in Nitrogen Fixation
... Since aminotransferases link the TCA cycle and amino acid synthesis, we expected they would have a major effect on nitrogen fixation. A mutant in the enzyme aspartate aminotransferase (aatA) formed defective nodules that are not able to fix nitrogen. The role of aatA would be the transamination of o ...
... Since aminotransferases link the TCA cycle and amino acid synthesis, we expected they would have a major effect on nitrogen fixation. A mutant in the enzyme aspartate aminotransferase (aatA) formed defective nodules that are not able to fix nitrogen. The role of aatA would be the transamination of o ...
Unit - 7.pmd
... covalency to four, nitrogen cannot form dπ –pπ bond as the heavier elements can e.g., R3P = O or R3P = CH2 (R = alkyl group). Phosphorus and arsenic can form dπ –dπ bond also with transition metals when their compounds like P(C2H5)3 and As(C6H5)3 act as ligands. (i) Reactivity towards hydrogen: All ...
... covalency to four, nitrogen cannot form dπ –pπ bond as the heavier elements can e.g., R3P = O or R3P = CH2 (R = alkyl group). Phosphorus and arsenic can form dπ –dπ bond also with transition metals when their compounds like P(C2H5)3 and As(C6H5)3 act as ligands. (i) Reactivity towards hydrogen: All ...
Descriptive Chemistry of Elements p
... We know that boron halides are electron deficient molecules and boron needs two more electrons to achieve the octet. Thus, these molecules can act as electron acceptors or Lewis acids. For example, ammonia (:NH3) and diethyl ether (Et2O:) donate a pair of lone-pair electrons to BF3 to form adducts H ...
... We know that boron halides are electron deficient molecules and boron needs two more electrons to achieve the octet. Thus, these molecules can act as electron acceptors or Lewis acids. For example, ammonia (:NH3) and diethyl ether (Et2O:) donate a pair of lone-pair electrons to BF3 to form adducts H ...
Effect of nitrogen fertilization on metabolisms of essential and non
... to cadmium, copper, and zinc (Hamer 1986). At the same time these proteins are cystein-rich proteins. This is again connected with the plant’s demand for available amino acids which are necessary for the production of proteins forming the aleurone layer (Chittenden et al. 1978). In terms of essentia ...
... to cadmium, copper, and zinc (Hamer 1986). At the same time these proteins are cystein-rich proteins. This is again connected with the plant’s demand for available amino acids which are necessary for the production of proteins forming the aleurone layer (Chittenden et al. 1978). In terms of essentia ...
1 chemistry of the nonmetals
... of energy is consumed in the United States.1 Less than 10% of this energy is provided by nuclear, solar, geothermal, or hydro power. The rest can be traced to a combustion reaction in which a fuel is oxidized by O2. The cars, trucks, and buses that fill our highways are powered by gasoline engines t ...
... of energy is consumed in the United States.1 Less than 10% of this energy is provided by nuclear, solar, geothermal, or hydro power. The rest can be traced to a combustion reaction in which a fuel is oxidized by O2. The cars, trucks, and buses that fill our highways are powered by gasoline engines t ...
Synthesis, identification and thermal decomposition of double
... the second step can be due to oxidation of Cu2O to CuO and/or oxidation of MIISO3 to MIISO4 probably by self-generated atmosphere formed by dense volatile products liberated in the latter step. # 2000 Elsevier Science B.V. All rights reserved. Keywords: Double sul®tes; Chevreul's salt; Thermal behav ...
... the second step can be due to oxidation of Cu2O to CuO and/or oxidation of MIISO3 to MIISO4 probably by self-generated atmosphere formed by dense volatile products liberated in the latter step. # 2000 Elsevier Science B.V. All rights reserved. Keywords: Double sul®tes; Chevreul's salt; Thermal behav ...
PROTEIN METABOLISM
... extracellular fluids is significantly lower than that within the cells of the body. This concentration gradient is maintained because ...
... extracellular fluids is significantly lower than that within the cells of the body. This concentration gradient is maintained because ...
Urea cycle
... • Ammonia originates in the catabolism of amino acids that are primarily produced by the degradation of proteins – dietary as well as existing within the cell: • digestive enzymes • proteins released by digestion of cells sloughed-off the walls of the GIT • muscle proteins • hemoglobin • intracellul ...
... • Ammonia originates in the catabolism of amino acids that are primarily produced by the degradation of proteins – dietary as well as existing within the cell: • digestive enzymes • proteins released by digestion of cells sloughed-off the walls of the GIT • muscle proteins • hemoglobin • intracellul ...
1 Introduction
... tracer allows for the simultaneous and repeated administration in one subject. Again, in paediatric investigations, this is no trivial advantage. Secondly, the mass spectrometry analysis of stable nuclide molecular position and isotopic enrichment in biological molecules is both highly specific and ...
... tracer allows for the simultaneous and repeated administration in one subject. Again, in paediatric investigations, this is no trivial advantage. Secondly, the mass spectrometry analysis of stable nuclide molecular position and isotopic enrichment in biological molecules is both highly specific and ...
Ch 18
... • An inborn error of metabolism results in the deficiency of argininosuccinase. What could be added to the diet to boost urea production aid in ammonia secretion? (Argininosuccinate can be excreted.) ...
... • An inborn error of metabolism results in the deficiency of argininosuccinase. What could be added to the diet to boost urea production aid in ammonia secretion? (Argininosuccinate can be excreted.) ...
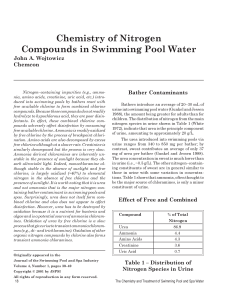
w_4-3 Chemistry of Nitrogen Compounds
... hydrolyze to hypochlorous acid, they are poor disinfectants. In effect, these combined chlorine compounds adversely affect disinfection by consuming free available chlorine. Ammonia is readily oxidized by free chlorine by the process of breakpoint chlorination. Amino acids are also decomposed by exc ...
... hydrolyze to hypochlorous acid, they are poor disinfectants. In effect, these combined chlorine compounds adversely affect disinfection by consuming free available chlorine. Ammonia is readily oxidized by free chlorine by the process of breakpoint chlorination. Amino acids are also decomposed by exc ...
The effecTs of benzoic acid and proTein level on urine ph and
... was as point of pH 0.88, which means up to 17 % (LPBA vs. HPBA). The decrease in urine pH (0.67 - 0.88 points of pH) is much lower than that (two points of pH decrease) obtained by Kristensen et al. (2009) in pigs of 63 ± 1 kg body weight. But Guingand et al. (2005) noted a decrease of 0.9 points as ...
... was as point of pH 0.88, which means up to 17 % (LPBA vs. HPBA). The decrease in urine pH (0.67 - 0.88 points of pH) is much lower than that (two points of pH decrease) obtained by Kristensen et al. (2009) in pigs of 63 ± 1 kg body weight. But Guingand et al. (2005) noted a decrease of 0.9 points as ...
Lecture Resource ()
... • Some amines are heterocyclic compounds (or heterocycles) • Most drugs, vitamins, and many other natural products are heterocycles • A natural product is a compound synthesized by a plant or an animal ...
... • Some amines are heterocyclic compounds (or heterocycles) • Most drugs, vitamins, and many other natural products are heterocycles • A natural product is a compound synthesized by a plant or an animal ...
Nitrogen Balance and Protein Requirements: Definition and
... dietary NEAA, despite the theoretical capability of the body to synthesise them, nitrogen will be needed for their de novo synthesis. This nitrogen in turn must be derived either from EAA catabolism (thus increasing their requirement above theoretical values) or from the diet. In this respect, altho ...
... dietary NEAA, despite the theoretical capability of the body to synthesise them, nitrogen will be needed for their de novo synthesis. This nitrogen in turn must be derived either from EAA catabolism (thus increasing their requirement above theoretical values) or from the diet. In this respect, altho ...
Plant and Soil
... review). However, the appearance of a covalently modified nitrogenase reductase during 'switch off" has clearly been demonstrated only in a few species (Gotto and Yoch, 1985; Hartmann et al., 1986; Jouanneau et al., 1983; Kanemoto and Ludden, 1984) (Fig. 2). The involvement of an activating (Saari e ...
... review). However, the appearance of a covalently modified nitrogenase reductase during 'switch off" has clearly been demonstrated only in a few species (Gotto and Yoch, 1985; Hartmann et al., 1986; Jouanneau et al., 1983; Kanemoto and Ludden, 1984) (Fig. 2). The involvement of an activating (Saari e ...
Isotope Fractionation: Why Aren`t We What We Eat?
... fractions listed in Table 1. The differences in the relative isotopic abundances of the elements that arise in two different molecular species or two different pools of the same molecular species that are observed in the biogeosphere are termed isotope fractionation. As indicated above, isotope frac ...
... fractions listed in Table 1. The differences in the relative isotopic abundances of the elements that arise in two different molecular species or two different pools of the same molecular species that are observed in the biogeosphere are termed isotope fractionation. As indicated above, isotope frac ...
p Block Elements General Configuration: ns2 np1
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
... Nitrogen differs from the rest of the members of this group due to its smaller size, high electro negativity, high ionization enthalpy and non-availability of d-orbitals. Nitrogen can form pπ-pπ multiple bond. Nitrogen exists as diatomic molecule with a triple bond. Heavier elements do not form pπ-p ...
Conservation of the metabolomic response to starvation across two divergent microbes.
... cells did stop before cells in complete minimal media (data not shown). The continued yeast growth in the absence of nitrogen also occurred in liquid culture, confirming that it is not an artifact of the filter-culture methodology (17). An implication of the increase in yeast biomass after removal o ...
... cells did stop before cells in complete minimal media (data not shown). The continued yeast growth in the absence of nitrogen also occurred in liquid culture, confirming that it is not an artifact of the filter-culture methodology (17). An implication of the increase in yeast biomass after removal o ...
Conservation of the metabolomic response to starvation across two divergent microbes.
... cells did stop before cells in complete minimal media (data not shown). The continued yeast growth in the absence of nitrogen also occurred in liquid culture, confirming that it is not an artifact of the filter-culture methodology (17). An implication of the increase in yeast biomass after removal o ...
... cells did stop before cells in complete minimal media (data not shown). The continued yeast growth in the absence of nitrogen also occurred in liquid culture, confirming that it is not an artifact of the filter-culture methodology (17). An implication of the increase in yeast biomass after removal o ...
Biology 20 Ch 3 Practice Test
... Scientists speculate that without life on earth, the composition of the atmosphere would be about 98% carbon dioxide. What biological process has greatly reduced the concentration of atmospheric carbon dioxide? a. Metabolism c. Respiration b. Photosynthesis d. Decomposition 6. The rate at which vari ...
... Scientists speculate that without life on earth, the composition of the atmosphere would be about 98% carbon dioxide. What biological process has greatly reduced the concentration of atmospheric carbon dioxide? a. Metabolism c. Respiration b. Photosynthesis d. Decomposition 6. The rate at which vari ...